2-巯基-3-吡啶醇 | 23003-22-7
中文名称
2-巯基-3-吡啶醇
中文别名
2-疏基-3-羟基吡啶;3-羟基-2(1H)-吡啶硫酮
英文名称
3-hydroxypyridine-2-thione
英文别名
3-hydroxypyridine-2(1H)-thione;3-hydroxy-pyridin-2-thione;2-sulfaniumylpyridin-3-olate
CAS
23003-22-7
化学式
C5H5NOS
mdl
MFCD00006286
分子量
127.167
InChiKey
MARYDOMJDFATPK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:141 °C
-
沸点:235.9±50.0 °C(Predicted)
-
密度:1.41±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:64.4
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2933399090
SDS
| Name: | 2-Mercapto-3-pyridinol 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 23003-22-7 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 23003-22-7 | 3-mercapto-3-pyridinol, 97% | 97 | 245-375-3 |
Risk Phrases:
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Appearance: yellow to green.
Not available.
Target Organs: None.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits +--------------------+-------------------+-------------------+-----------------+ | Chemical Name | ACGIH | NIOSH |OSHA - Final PELs| |--------------------|-------------------|-------------------|-----------------| | 3-mercapto-3-pyridi|none listed |none listed |none listed | | nol, 97% | | | | +--------------------+-------------------+-------------------+-----------------+ OSHA Vacated PELs: 3-mercapto-3-pyridinol, 97%: No OSHA Vacated PELs are listed for this chemical.
Personal Protective Equipment Eyes: Wear chemical goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant a respirator's use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Appearance: yellow to green
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: @ 760.00mm Hg
Freezing/Melting Point: 144.00 - 146.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
NFPA Rating: Not published.
Explosion Limits, Lower: Not available.
Upper: Not available.
Decomposition Temperature:
Solubility:
Specific Gravity/Density:
Molecular Formula: C5H5NOS
Molecular Weight: 127.16
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 23003-22-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-mercapto-3-pyridinol, 97% - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA.
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Chemical waste generators must determine whether a discarded chemical is classif as a hazardous waste.
US EPA guidelines for the classification determination are listed in 40 CFR Part Additionally, waste generators must consult state and local hazardous waste regu ensure complete and accurate classification.
RCRA P-Series: None listed.
RCRA U-Series: None listed.
Section 14 - TRANSPORT INFORMATION
CDG/CPL
Not classified as hazardous for supply.
Canadian TDG
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 23003-22-7: No information available.
United Kingdom Occupational Exposure Limits
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
WHMIS: Not available.
CAS# 23003-22-7 is not listed on Canada's Ingredient Disclosure List.
Exposure Limits
US FEDERAL
TSCA
CAS# 23003-22-7 is not listed on the TSCA inventory.
It is for research and development use only.
Health & Safety Reporting List
None of the chemicals are on the Health & Safety Reporting List.
Chemical Test Rules
None of the chemicals in this product are under a Chemical Test Rule.
Section 12b
None of the chemicals are listed under TSCA Section 12b.
TSCA Significant New Use Rule
None of the chemicals in this material have a SNUR under TSCA.
SARA
Section 302 (RQ)
None of the chemicals in this material have an RQ.
Section 302 (TPQ)
None of the chemicals in this product have a TPQ.
Section 313
No chemicals are reportable under Section 313.
Clean Air Act:
This material does not contain any hazardous air pollutants.
This material does not contain any Class 1 Ozone depletors.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲硫基吡啶-3-醇 2-(methylsulfanyl)pyridin-3-ol 32637-37-9 C6H7NOS 141.194 —— 2-isopropylthio-3-hydroxypyridine 145099-52-1 C8H11NOS 169.247
反应信息
-
作为反应物:描述:2-巯基-3-吡啶醇 在 anionic resin Amberlite IRA-401 作用下, 以 水 、 甲苯 为溶剂, 反应 48.0h, 生成 2,3-二氢噻唑并[3,2-a]吡啶鎓-8-醇内盐参考文献:名称:Katritzky, Alan R.; Grzeskowiak, Nicholas E., Journal of Chemical Research, Miniprint, 1981, # 7, p. 2345 - 2389摘要:DOI:
-
作为产物:描述:参考文献:名称:Katritzky, Alan R.; Grzeskowiak, Nicholas E., Journal of Chemical Research, Miniprint, 1981, # 7, p. 2345 - 2389摘要:DOI:
文献信息
-
Thionations Using a P<sub>4</sub>S<sub>10</sub>−Pyridine Complex in Solvents Such as Acetonitrile and Dimethyl Sulfone作者:Jan Bergman、Birgitta Pettersson、Vedran Hasimbegovic、Per H. SvenssonDOI:10.1021/jo101865y日期:2011.3.18Tetraphosphorus decasulfide (P4S10) in pyridine has been used as a thionating agent for a long period of time. The moisture-sensitive reagent has now been isolated in crystalline form, and the detailed structure has been determined by X-ray crystallography. The thionating power of this storable reagent has been studied and transferred to solvents such as acetonitrile in which it has proven to be synthetically四硫化二磷(P 4 S 10)中的吡啶已长期用作硫代剂。现在已经以结晶形式分离出对水分敏感的试剂,并且已经通过X射线晶体学确定了详细的结构。已经研究了这种可储存试剂的去硫能力,并将其转移到诸如乙腈之类的溶剂中,在该溶剂中已证明其在合成上是有用的并且具有非凡的选择性。它的性能已经与所谓的Lawesson试剂(LR)进行了比较。特别有趣的是在相对较高的温度(约165°C)下以二甲基砜为溶剂进行硫磺化的结果。在这些条件下,例如,a啶酮和3-乙酰吲哚可以迅速转化为相应的亚硫代衍生物。甘氨酰甘氨酸类似地得到哌嗪去甲酮。在这些温度下 LR由于快速分解而效率低下。硫代产物通常更清洁并且更容易获得,因为在结晶试剂中,常规试剂P中始终存在杂质吡啶或LR中的4 S 10已被除去。
-
Tetrazole derivatives, and anti-ulcer composition containing the same申请人:Otsuka Pharmaceutical Company, Limited公开号:US04372953A1公开(公告)日:1983-02-08Tetrazole derivatives of the formula: ##STR1## wherein R.sup.1 is a lower alkykl, phenyl or a group of the formula: --S(O).sub.l --A--(X).sub.m --R.sup.3, and R.sup.2 is hydrogen, a lower alkyl, phenyl or a cycloalkyl when R.sup.1 is the group --S(O).sub.l --A--(X).sub.m --R.sup.3, or R.sup.2 is a group of the formula: --B--CO--R.sup.4 when R.sup.1 is a lower alkyl or phenyl and a pharmaceutically acceptable salt thereof, which have prophylactic or therapeutic activities against peptic and/or duodenal ulcers and are useful as an anti-ulcer drug; processes for the preparation of the tetrazole derivatives; and pharmaceutical composition containing said tetrazole derivatives.Tetrazole衍生物的化学式为:##STR1## 其中R.sup.1是较低的烷基、苯基或化学式的基团:--S(O).sub.l --A--(X).sub.m --R.sup.3,而R.sup.2是氢、较低的烷基、苯基或环烷基,当R.sup.1是基团--S(O).sub.l --A--(X).sub.m --R.sup.3时,或者R.sup.2是化学式的基团:--B--CO--R.sup.4,当R.sup.1是较低的烷基或苯基时,以及其药学上可接受的盐,具有预防或治疗胃溃疡和/或十二指肠溃疡的活性,并可用作抗溃疡药物;制备Tetrazole衍生物的方法;以及含有上述Tetrazole衍生物的药物组合物。
-
Photo-induced Carbon-Carbon Bond Formation: Reactions of 2-Thiopyridones and 2-Quinolinethiones with Alkenes作者:Takehiko Nishio、Yoshimori OmoteDOI:10.1055/s-1986-31475日期:——A synthetically useful method for the formation of C-C bonds involving photochemically induced addition of 2-thioxo-1,2-dihydropyridines or -quinolines to alkenes is described. The products are 2-(2-mercaptoalkyl)-pyridines and -quinolines.
-
Synthesis and Antitumor Activity of Novel Mitomycin Derivatives Containing Functional Groups at the C-6-Methyl Position作者:Hitoshi Arai、Yutaka Kanda、Tadashi Ashizawa、Makoto Morimoto、Katsushige Gomi、Motomichi Kono、Masaji KasaiDOI:10.1021/jm00038a008日期:1994.6compounds exhibited remarkable activity against MMC-resistant P388 in mice. FAB-MS spectra of these mitomycin derivatives showed the elimination of the C-6-methyl substituents from the mitomycin skeletons to form quinonemethides. Interestingly, treatment of 6-demethyl-6-[[(2-pyrimidinyl)thio]methyl ]mitomycin C (12v) with diethylamine afforded 6-demethyl-6-[(diethylamino)methyl]mitomycin C (31) in good合成了一系列C-6取代的甲基丝裂霉素,并评估了其抗细胞和抗肿瘤活性。这些新颖的化合物是通过将各种醇或硫醇迈克尔加成至6-去甲基-7,7-(乙二氧基)-6,7-二氢-6-亚甲基亚神经松脂中,然后用NH3或MeOH / K2CO3处理而制备的。大多数化合物对HeLa S3均有效,其中一些化合物对小鼠的P388白血病和肉瘤180显示出优于丝裂霉素C(MMC)的活性。此外,某些化合物对小鼠的MMM耐药P388表现出显着活性。这些丝裂霉素衍生物的FAB-MS光谱表明,从丝裂霉素骨架上消除了C-6-甲基取代基,从而形成了醌甲基化物。有趣的是 用二乙胺处理6-去甲基-6-[[[(2-嘧啶基)硫代]甲基]丝裂霉素C(12v)得到6-脱甲基-6-[[(二乙氨基)甲基]丝裂霉素C(31),收率高。这些结果表明,C-6取代的甲基丝裂霉素将具有与MMC不同的生物学特性。
-
Antitrichomonal activity of mesoionic thiazolo[3,2-a]pyridines作者:Keith A. M. Walker、Eric B. Sjogren、Thomas R. MatthewsDOI:10.1021/jm00149a023日期:1985.11Screening of mesoionic compounds as potential electron acceptors by analogy with metronidazole led to the finding of in vitro antitrichomonal activity for anhydro-2-phenyl-3-hydroxythiazolo [3,2-a]pyridinium hydroxide (1). In a series of analogues, potent in vitro activity was found to be associated with amino substitution; however, such activity was dependent on specific structural features and not
表征谱图
-
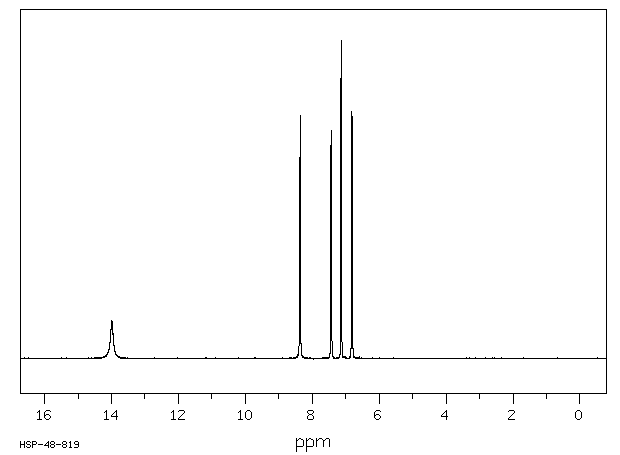
氢谱1HNMR
-
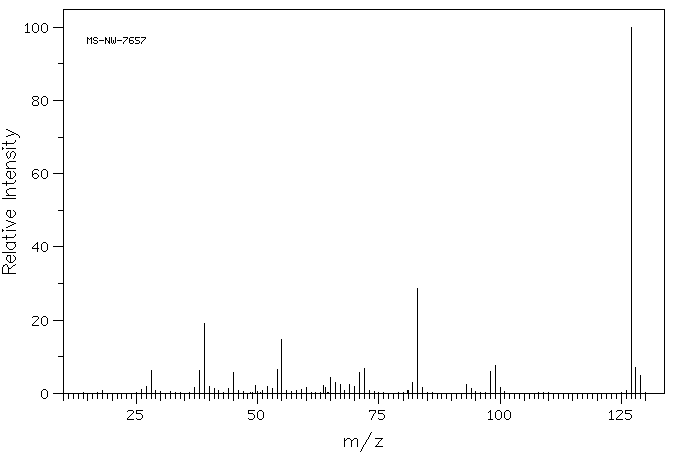
质谱MS
-
碳谱13CNMR
-
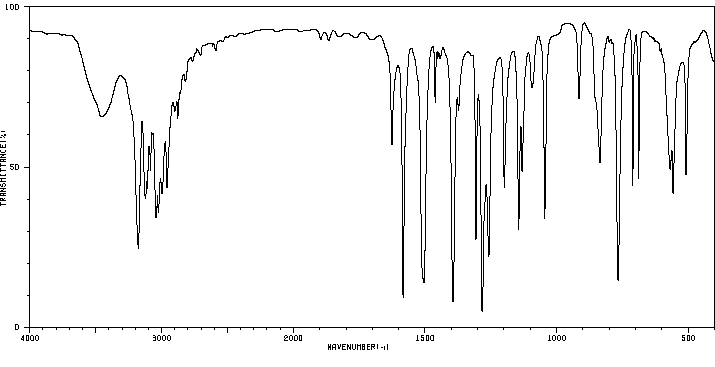
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-