2-羟基乙基异丙基硫醚 | 40811-49-2
中文名称
2-羟基乙基异丙基硫醚
中文别名
2-异丙基巯基乙醇;2-(异丙基硫基)乙醇
英文名称
2-(isopropylthio)ethanol
英文别名
2-propan-2-ylsulfanylethanol
CAS
40811-49-2
化学式
C5H12OS
mdl
MFCD00014038
分子量
120.216
InChiKey
QGONIKZRWOOHBB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:110-111°C 30mm
-
密度:0,979 g/cm3
-
闪点:110-111°C/30mm
-
稳定性/保质期:
常规情况下不会分解,也没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:45.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:6.1
-
安全说明:S23,S36/37
-
危险类别码:R20/21/22
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:UN 2810
-
储存条件:密封、阴凉、干燥处保存。
SDS
2-(异丙基硫基)乙醇 修改号码:5
模块 1. 化学品
产品名称: 2-(Isopropylthio)ethanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-(异丙基硫基)乙醇
百分比: >95.0%(GC)
CAS编码: 40811-49-2
俗名: 2-Hydroxyethyl Isopropyl Sulfide
分子式: C5H12OS
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
2-(异丙基硫基)乙醇 修改号码:5
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 96 °C/3.2kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.98
溶解度:
[水] 无资料
[其他溶剂] 无资料
2-(异丙基硫基)乙醇 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2-(异丙基硫基)乙醇 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-(Isopropylthio)ethanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-(异丙基硫基)乙醇
百分比: >95.0%(GC)
CAS编码: 40811-49-2
俗名: 2-Hydroxyethyl Isopropyl Sulfide
分子式: C5H12OS
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
2-(异丙基硫基)乙醇 修改号码:5
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 96 °C/3.2kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.98
溶解度:
[水] 无资料
[其他溶剂] 无资料
2-(异丙基硫基)乙醇 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2-(异丙基硫基)乙醇 修改号码:5
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:2-羟基乙基异丙基硫醚 在 氢氧化钾 作用下, 以48%的产率得到2-乙烯基硫代-丙烷参考文献:名称:“远程”烷基的意外立体效应对烷基α,β-乙烯砜,亚砜和酯的共轭加成速率摘要:在迈克尔型加成反应中对共轭乙烯砜,亚砜和酯的检测首次显示,杂原子连接的烷基的大小会影响共轭添加的速率。分子建模有力地表明,什么通常被认为是“远程”中的烷基-C β ħ Ç α HS(O)ñ烷基系统和-CH 2 C ^ β ħ Ç α HCOO烷基系统实际上是从β-不远程迈克尔接受单元的碳原子。分子模型清楚地表明,这些迈克尔受体中的烷基按以下顺序屏蔽了β-碳:Et < i -Pr < t-Bu 竞争实验确定迈克尔加成的相对比率按以下顺序排列:Et> i -Pr> t -Bu。DOI:10.1021/jo062155g
-
作为产物:描述:参考文献:名称:1,3-氧杂硫杂环戊烷与氢化二异丁基铝的裂解摘要:DOI:10.1007/bf00546752
文献信息
-
On the reported intermediacy of vinyl radicals in spontaneous polymerization: An ESR-spin trapping study and its significance for the bond forming initiation theory作者:Eugene A. Mash、Hans-Gert Korth、Suzanne M. DeMossDOI:10.1016/s0040-4020(97)00964-2日期:1997.11Deuterated isopropyl vinyl sulfides and diethyl fumarate were synthesized and employed in a re-investigation of the mechanism of initiation of spontaneous polymerization of these comonomers by means of spin-trapping/ESR spectroscopy. Previous radical spin-trapping studies had been interpreted as indicating the involvement of vinyl radicals. While our studies produced data substantially in agreement
-
[EN] REV-ERB AGONISTS FOR THE TREATMENT OF TH17-MEDIATED INFLAMMATORY DISORDERS<br/>[FR] AGONISTES REV-ERB POUR LE TRAITEMENT DE TROUBLES INFLAMMATOIRES À MÉDIATION PAR TH17申请人:SCRIPPS RESEARCH INST公开号:WO2021263278A1公开(公告)日:2021-12-30The present disclosure provides compounds of Formula IA and Formula IB and their pharmaceutical compositions as selective agonists of REV-ERB-α: where R1, R2, R3, R4, R5, RX1, RX2, nA, nB, X, Y, and Z are described herein. The compounds are useful in various methods and uses, such as in the treatment of diseases including hyperglycemia, dyslipidemia, atherosclerosis, and autoimmune and inflammatory disorders or diseases, and as cancer therapeutics, such as for the treatment of glioblastoma, hepatocellular carcinoma, and colorectal cancer, and for immune-oncology purposes.本公开提供了Formula IA和Formula IB的化合物及其作为REV-ERB-α选择性激动剂的药物组合物:其中R1、R2、R3、R4、R5、RX1、RX2、nA、nB、X、Y和Z如本文所述。这些化合物在各种方法和用途中非常有用,例如用于治疗包括高血糖、血脂异常、动脉粥样硬化、自身免疫和炎症性疾病等疾病,以及作为癌症治疗药物,如用于治疗胶质母细胞瘤、肝细胞癌和结直肠癌,以及用于免疫肿瘤学目的。
-
Synthesis of N-monosubstituted S-aminoethyl hydrogen thiosulphates with sulphone substituents
-
The reductive fission of methyl sulphides, 1,3-dithiolans, and a 1,3-oxathiolan by sodium in liquid ammonia作者:E. D. Brown、S. M. Iqbal、L. N. OwenDOI:10.1039/j39660000415日期:——3-dimercaptopropanol can be regenerated from its SS-methylene derivative, but not from its SS-isopropylidene derivative, by sodium in liquid ammonia, is explained by the difference in stability of the respective intermediate alkylthio-derivatives. In a series of 2-(alkylthio)ethanols, only the methylthio-compound is cleaved. The isopropylidene derivatives of cyclohexane-trans-1,2-dithiol, 2-mercaptocyclohexanol
-
Catalytic Asymmetric Csp3 −H Functionalization under Photoredox Conditions by Radical Translocation and Stereocontrolled Alkene Addition作者:Chuanyong Wang、Klaus Harms、Eric MeggersDOI:10.1002/anie.201607305日期:2016.10.17photoredox‐mediated C(sp3)−H activation through radical translocation can be combined with asymmetric catalysis. Upon irradiation with visible light, α,β‐unsaturated N‐acylpyrazoles react with N‐alkoxyphthalimides in the presence of a rhodium‐based chiral Lewis acid catalyst and the photosensitizer fac‐[Ir(ppy)3] to provide a C−C bond‐formation product with high enantioselectivity (up to 97 % ee) and, where applicable
表征谱图
-
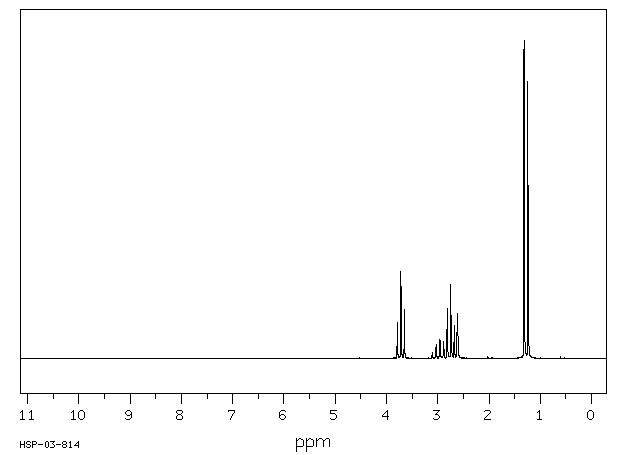
氢谱1HNMR
-
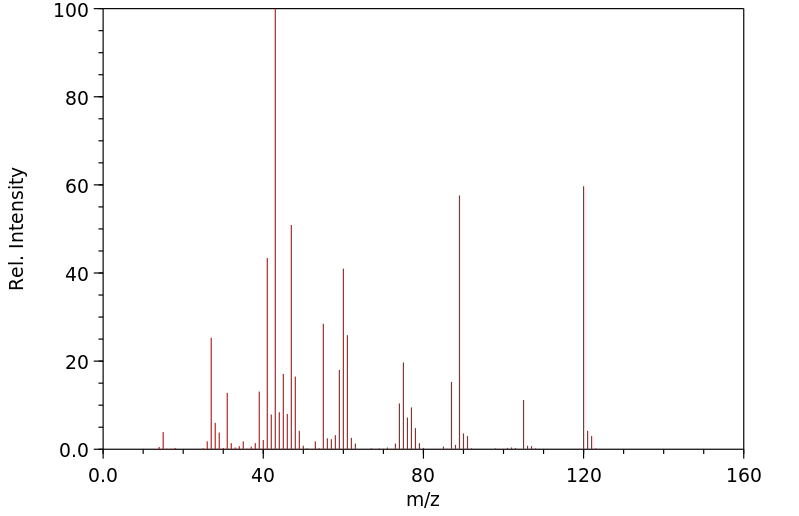
质谱MS
-
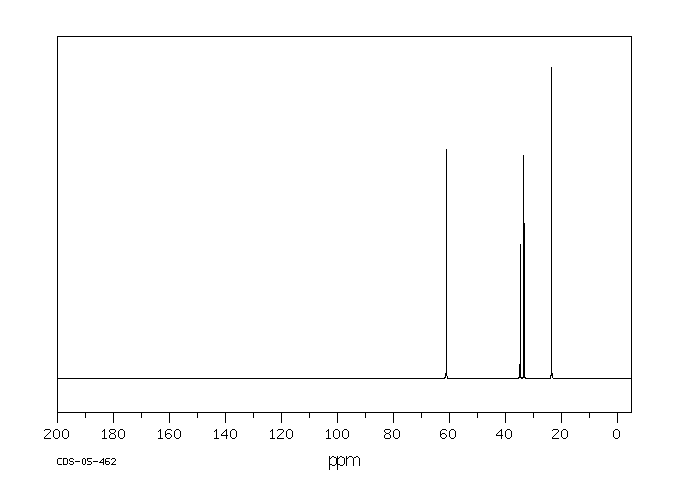
碳谱13CNMR
-
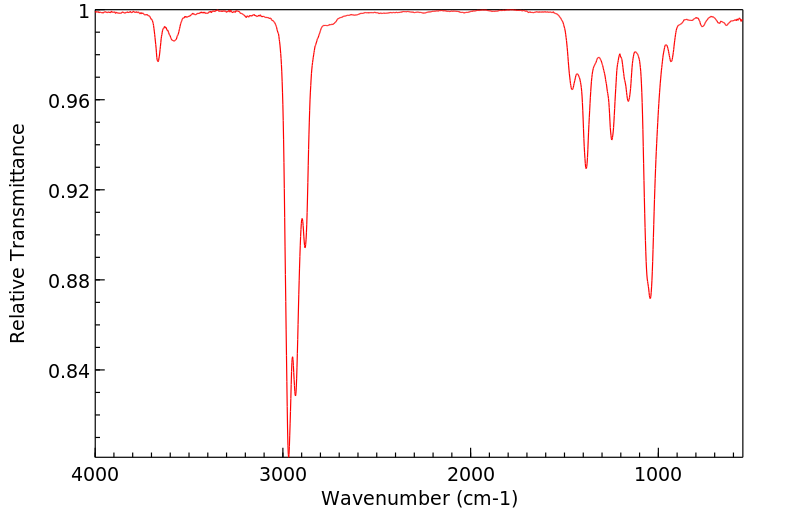
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯