3-(4-甲氧基苯基)-1,1-二甲基硫脲 | 31124-88-6
中文名称
3-(4-甲氧基苯基)-1,1-二甲基硫脲
中文别名
——
英文名称
3-(4-methoxyphenyl)-1,1-dimethylthiourea
英文别名
——
CAS
31124-88-6
化学式
C10H14N2OS
mdl
MFCD00025793
分子量
210.3
InChiKey
JPDXJCNMWXNPGV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:14
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:56.6
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2930909090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Utsumi et al., Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1954, vol. 74, p. 238,240摘要:DOI:
-
作为产物:描述:参考文献:名称:Use of Tetramethylthiuram Disulfide in Synthesis of Nitrogen-containing Heterocyclic Compounds摘要:DOI:10.1007/s10593-005-0200-x
文献信息
-
Facile synthesis of S -arylisothioureas via the S -arylation of arylthioureas with aryl iodides in water作者:Xing Liu、Hui Zhu、Shi-Bo Zhang、Yu Cheng、Han-Ying Peng、Zhi-Bing DongDOI:10.1016/j.tetlet.2018.07.012日期:2018.8An environmentally benign, simple and highly efficient protocol for the synthesis of S-arylisothiourea derivatives has been achieved in good to excellent yields by reacting a series of aryl iodides with arylthioureas, using inexpensive CuSO4·5H2O as catalyst in water without PTC (phase transfer catalyst). The protocol features easy performance, good to excellent yields, good tolerance towards a variety
-
Copper-Catalyzed S-Arylation of Arylthioureas by Using Diaryliodonium Salts作者:Yue-Sheng Li、Zhi-Bing Dong、Hui Zhu、Xing Liu、Yu Cheng、Han-Ying PengDOI:10.1055/s-0036-1591569日期:2018.6and pharmaceutical compounds. A convenient and efficient copper-catalyzed S-arylation of arylthioureas using diaryliodonium salts is reported. The desired arylisothioureas were synthesized in good yields in the presence of CuCl as catalyst and K2CO3 as base, and a wide variety of functional groups on the arylthioureas and diaryliodonium salts were tolerated. The protocol affords an alternative synthesis
-
A Mild Synthesis of 2-Substituted Benzothiazoles via Nickel-Catalyzed Intramolecular Oxidative C–H Functionalization作者:Ming-Yuan Gao、Jing-Hang Li、Shi-Bo Zhang、Li-Jun Chen、Yue-Sheng Li、Zhi-Bing DongDOI:10.1021/acs.joc.9b02543日期:2020.1.17A highly efficient synthetic method for the preparation of 2-aminobenzothiazoles starting from arylthioureas has been reported. By using a nickel catalyst, arylthioureas undergo intramolecular oxidative C-H bond functionalization, giving the desired 2-aminobenzothiazoles in good to excellent yields. This protocol features an inexpensive catalyst, low catalyst loading, mild reaction conditions, a short
-
An Efficient Chan-Lam <i>S</i> -Arylation of Arylthioureas with Arylboronic Acids作者:Xing Liu、Shi-Bo Zhang、Hui Zhu、Yu Cheng、Han-Ying Peng、Zhi-Bing DongDOI:10.1002/ejoc.201800892日期:2018.8.31A convenient and efficient protocol has been developed for the preparation of aryl-isothioureas based on the Chan-Lam C-S coupling reaction. The desired aryl-isothioureas were synthesized in good yields (up to 95%) in the presence of Cu(OAc)(2)H2O as catalyst and bipyridine as ligand. A variety of functional groups on the aryl-thiourea and arylboronic acid reagents are tolerated. The method features
-
Insertion of Arynes into Thioureas: A New Amidine Synthesis作者:Kallolmay Biswas、Michael F. GreaneyDOI:10.1021/ol202049v日期:2011.9.16thioureas via a formal π-insertion into the C═S bond. The reaction contrasts with that of ureas, which proceeds via benzyne σ-insertion into the C–N bond, and represents a new, operationally simple route to functionalized amidines.
表征谱图
-
氢谱1HNMR
-
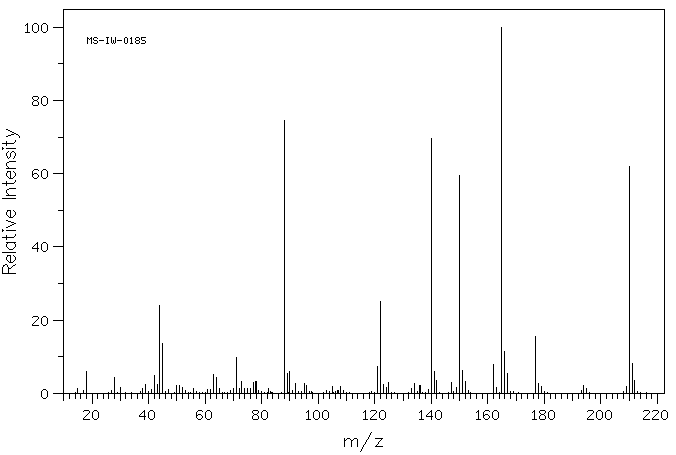
质谱MS
-
碳谱13CNMR
-
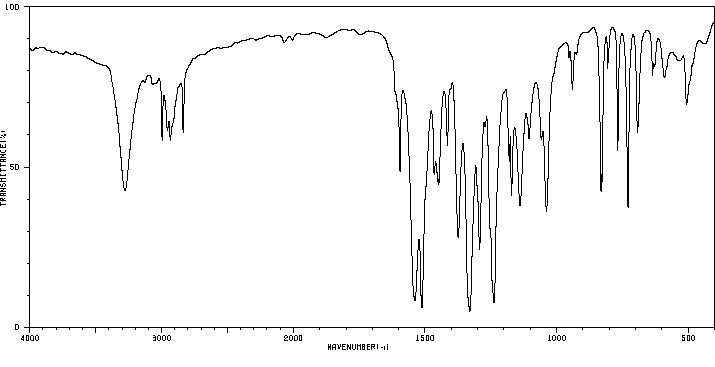
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫