二苯基-2-吡啶基甲烷 | 3678-70-4
中文名称
二苯基-2-吡啶基甲烷
中文别名
联苯-2-吡啶甲烷
英文名称
Diphenyl-2-pyridylmethan
英文别名
Diphenyl-2-pyridylmethane;2-benzhydrylpyridine
CAS
3678-70-4
化学式
C18H15N
mdl
——
分子量
245.324
InChiKey
MMXYNKLYVRNTCK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:54-61°C
-
沸点:378.28°C (rough estimate)
-
密度:0.8855 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:19
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
海关编码:2933399090
SDS
| Name: | Diphenyl-2-Pyridylmethane 99+% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 3678-70-4 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3678-70-4 | Diphenyl-2-Pyridylmethane | 99+ | 222-953-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3678-70-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: light yellow
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 59.00 - 62.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C18H15N
Molecular Weight: 245.32
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3678-70-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Diphenyl-2-Pyridylmethane - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 3678-70-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3678-70-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3678-70-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-苄基吡啶 2-Benzylpyridine 101-82-6 C12H11N 169.226 吡啶-2-YL二苯基甲醇 diphenyl(2-pyridyl)methanol 19490-90-5 C18H15NO 261.323 —— 2-pyridynyldiphenylacetonitrile 83695-12-9 C19H14N2 270.334 1-苯基-1-(吡啶-2-基)甲醇 phenyl(2-pyridinyl)methanol 31796-72-2 C12H11NO 185.225 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(1-phenylbenzyl)pyridine N-oxide 21883-34-1 C18H15NO 261.323 —— 2-(1,1-diphenylethyl)pyridine 24187-15-3 C19H17N 259.351 吡啶-2-YL二苯基甲醇 diphenyl(2-pyridyl)methanol 19490-90-5 C18H15NO 261.323 (吡啶-2-基)二苯基氯甲烷 diphenyl-(2-pyridyl)methyl chloride 53608-51-8 C18H14ClN 279.769 —— dichloro-[diphenyl(pyridin-2-yl)methyl]silane 130710-20-2 C18H15Cl2NSi 344.315
反应信息
-
作为反应物:描述:参考文献:名称:The Absorption Spectra of Some Methylpyridine Derivatives摘要:DOI:10.1021/ja01169a098
-
作为产物:描述:参考文献:名称:前所未有的带有六元螯合三齿C ^ N ^ C配体的发光铱(III)配合物家族摘要:一个新的家族,由三个发光的中性Ir(III)配合物组成,具有前所未有的[Ir(C ^ N ^ C)(N ^ N)Cl]结构,其中C ^ N ^ C是双(六元)螯合三齿三脚架的配体由2- benzhydrylpyridine(bnpy)和N 1,N衍生是4,4'-二-叔丁基-2,2'-联吡啶(d吨Bubpy),则报告。X射线晶体学分析显示,bnpy的两个远端非共轭苯环具有意想不到且异常的双C–H键活化,加上非常短的Ir–Cl键反式bnpy配体的吡啶。根据在bnpy配体上的取代,在二氯甲烷溶液中观察到磷光,范围从黄色到红色。结合使用密度泛函理论(DFT)和时变DFT(TD-DFT)的研究证实了相关激发态的混合电荷转移性质。DOI:10.1021/acs.inorgchem.7b00328
文献信息
-
Synthesis of Triarylmethanes via Palladium-Catalyzed Suzuki–Miyaura Reactions of Diarylmethyl Esters作者:Amira H. Dardir、Irene Casademont-Reig、David Balcells、Jonathan D. Ellefsen、Matthew R. Espinosa、Nilay Hazari、Nicholas E. SmithDOI:10.1021/acs.organomet.1c00085日期:2021.7.26compatible with systems for diarylmethyl ester coupling. Furthermore, the reaction can be performed stereospecifically to generate stereoinverted products. On the basis of DFT calculations, it is proposed that the oxidative addition of the diarylmethyl 2,3,4,5,6-pentafluorobenzoate substrate occurs via an SN2 pathway, which results in the inverted products. Mechanistic studies indicate that oxidative addition描述了通过 Pd 催化的 2,3,4,5,6-五氟苯甲酸二芳基甲酯和芳基硼酸之间的 Suzuki-Miyaura 反应合成三芳基甲烷。该系统在温和的条件下运行,具有广泛的底物范围,包括不包含扩展芳族取代基的二苯甲醇衍生物的偶联。这一点很重要,因为这些底物会导致药物中普遍存在的三芳基甲烷产物类型,而这些底物以前并不与二芳基甲酯偶联系统兼容。此外,该反应可以立体特异性地进行以产生立体反转产物。在 DFT 计算的基础上,提出 2,3,4,5,6-五氟苯甲酸二芳基甲酯底物的氧化加成是通过 S N2 通路,从而产生倒置产物。机理研究表明,将 2,3,4,5,6-五氟苯甲酸二芳基甲酯底物氧化加成到 (IPr)Pd(0) 会导致 O-C(苄基) 键的选择性断裂,部分原因是稳定的 η苄基配体与 Pd 之间的3 -相互作用。这与先前描述的涉及苯基酯的 Pd 催化的 Suzuki-Miyaura 反应形成对比,后者涉及
-
Hydrogenation of Pyridines Using a Nitrogen-Modified Titania-Supported Cobalt Catalyst作者:Feng Chen、Wu Li、Basudev Sahoo、Carsten Kreyenschulte、Giovanni Agostini、Henrik Lund、Kathrin Junge、Matthias BellerDOI:10.1002/anie.201803426日期:2018.10.26Novel heterogeneous catalysts were prepared by impregnation of titania with a solution of cobalt acetate/melamine and subsequent pyrolysis. The resulting materials show an unusual nitrogen‐modified titanium structure through partial implementation of nitrogen into the support. The optimal catalyst displayed good activity and selectivity for challenging pyridine hydrogenation under acid free conditions
-
Palladium-Catalyzed Arylation of Benzylic C–H Bonds of Azaarylmethanes with Aryl Sulfides作者:Ke Gao、Keita Yamamoto、Keisuke Nogi、Hideki YorimitsuDOI:10.1055/s-0036-1589098日期:2017.12Benzylic C–H arylation of azaarylmethanes with aryl sulfides has been developed by using a Pd-NHC catalyst and an amide base. Various azaarylmethanes and aryl sulfides were involved in the reaction to afford the corresponding diarylmethanes in good to excellent yields. Moreover, triarylmethane synthesis was accomplished through iterative arylations of 2- or 4-methylpyridine with two different aryl
-
Iron-Catalyzed C-H Bond Functionalization for the Exclusive Synthesis of Pyrido[1,2-<i>a</i>]indoles or Triarylmethanols作者:Iyyanar Karthikeyan、Govindasamy SekarDOI:10.1002/ejoc.201403233日期:2014.12The efficient and selective iron-catalyzed C–H activation of 2-benzhydrylpyridine derivatives was employed for the preparation of pyrido[1,2-a]indoles through an intramolecular C–H amination reaction. In the presence of molecular oxygen as the sole oxidant, the same 2-benzhydrylpyridines were also used for the synthesis of the corresponding tertiary alcohols. In these approaches, the iron catalyst
-
Enantio‐ and Regioselective Palladium(II)‐Catalyzed Dioxygenation of (Aza‐)Alkenols作者:Sabrina Giofrè、Letizia Molteni、Donatella Nava、Leonardo Lo Presti、Egle Maria BeccalliDOI:10.1002/anie.202109312日期:2021.9.27An oxidative Pd-catalyzed intra-intermolecular dioxygenation of (aza-)alkenols has been reported, with total regioselectivity. To study the stereoselectivity, different chiral ligands as well as different hypervalent-iodine compounds have been compared. In particular, by using a C-6 modified pyridinyl-oxazoline (Pyox) ligand and hypervalent iodine bearing an aromatic ring, an excellent enantio- and
表征谱图
-
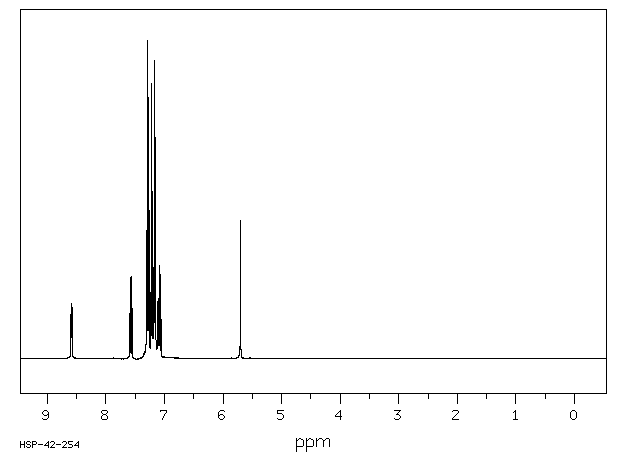
氢谱1HNMR
-
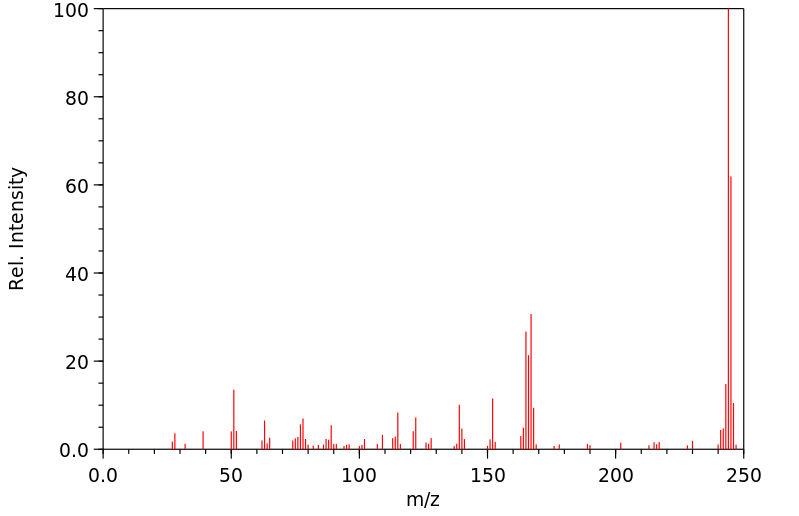
质谱MS
-
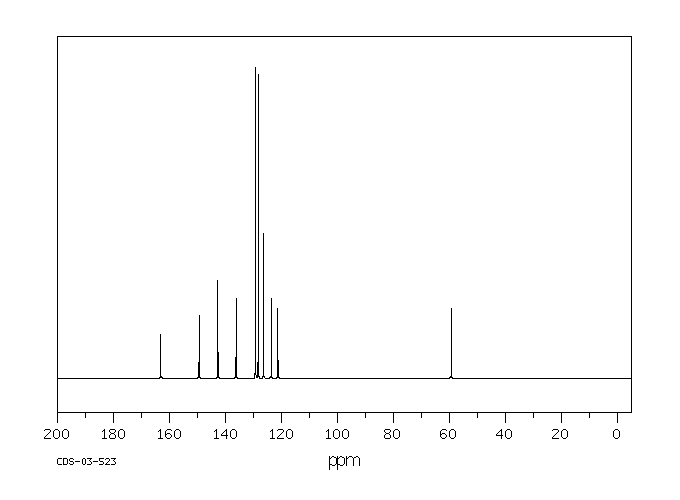
碳谱13CNMR
-
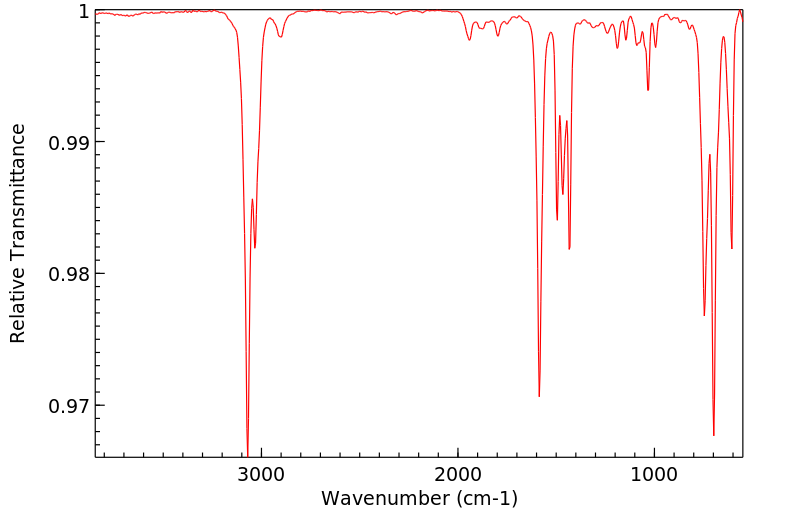
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫