甲基三(三甲基硅氧烷基)硅烷 | 17928-28-8
中文名称
甲基三(三甲基硅氧烷基)硅烷
中文别名
——
英文名称
methyltrimethicone
英文别名
methyltris(trimethylsiloxy)silane;trimethyl-[methyl-bis(trimethylsilyloxy)silyl]oxysilane
CAS
17928-28-8
化学式
C10H30O3Si4
mdl
MFCD00040007
分子量
310.688
InChiKey
RGMZNZABJYWAEC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-76°C
-
沸点:60 °C
-
密度:0.849
-
闪点:61°C
-
LogP:8.2 at 20℃
-
物理描述:Methyltris(trimethylsiloxy)silane is a clear colorless liquid. (NTP, 1992)
-
溶解度:less than 1 mg/mL at 68° F (NTP, 1992)
-
蒸汽压力:0.04 mmHg
-
保留指数:1010.2
计算性质
-
辛醇/水分配系数(LogP):5.01
-
重原子数:17
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S16,S26,S36,S36/37/39
-
危险类别码:R36/37/38
-
危险品运输编号:1993
-
海关编码:2934999090
-
包装等级:III
-
危险类别:3
-
危险性防范说明:P210,P403+P235
-
危险性描述:H225
-
储存条件:存放于惰性气体中,并避免接触湿气(否则可能分解)。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1,1,1,3,5,5,5-heptamethyltrisiloxanol 5272-21-9 C7H22O3Si3 238.506 双(三甲基硅氧基)甲基甲氧基硅烷 3-methoxyheptamethyltrisiloxane 7671-19-4 C8H24O3Si3 252.533 双三甲基硅氧基甲基硅烷 1,1,1,3,5,5,5-heptamethyltrisiloxan 1873-88-7 C7H22O2Si3 222.507 —— Ethoxy-methyl-bis-trimethylsilyloxy-silan 17908-27-9 C9H26O3Si3 266.56
反应信息
-
作为反应物:描述:参考文献:名称:Voronkov, M. G.; Basenko, S. V.; Ustinov, M. V., Doklady Chemistry, 1993, vol. 333, # 1-6, p. 273 - 276摘要:DOI:
-
作为产物:描述:参考文献:名称:Mueller et al., Journal fur praktische Chemie (Leipzig 1954), 1959, vol. <4>9, p. 63,67,70摘要:DOI:
文献信息
-
Pd/C-catalyzed cross-coupling reaction of benzyloxysilanes with halosilanes for selective synthesis of unsymmetrical siloxanes作者:Masayasu Igarashi、Keiko Kubo、Tomohiro Matsumoto、Kazuhiko Sato、Wataru Ando、Shigeru ShimadaDOI:10.1039/c4ra02126f日期:——A new protocol for the nonhydrolytic synthesis of unsymmetrical siloxanes has been developed. The cross-coupling reaction of benzyloxysilanes with halosilanes catalyzed by Pd/C afforded various unsymmetrical siloxanes with co-production of benzyl halides.
-
Synthesis of siloxanes作者:U. Scheim、R. Lehnert、A. Porzel、K. RühlmannDOI:10.1016/0022-328x(88)83083-3日期:1988.11NMR spectroscopy has been used in a kinetic study of the cleavage of siloxane bonds by hydrogen chloride in dioxane. The cleavages show an induction period which is associated with the autocatalytic effect of the water formed during the reaction. The kinetic behavior can be expressed in terms of a rate law that includes rate contants for cleavage by both dioxane. HCl (k1) and H2O·HCl(k2). The k′1 (k′1
-
Zur Synthese von Siloxanen作者:U. Scheim、A. Porzel、K. RühlmannDOI:10.1016/0022-328x(88)80636-3日期:1988.10The cleavage of siloxane bonds by BF3 was investigated kinetically by 1H NMR spectroscopy. The reactions could be evaluated by use of a pseudo-first order rate law, when a sufficiently large excess of BF3 was used. The order with respect to BF3 was found to be 4.7. The rate constants obtained could be correlated to σ*-values with a ϱ*-value of −1.0. The σ*-value found previously by 29Si NMR spectroscopy通过1 H NMR光谱动力学研究了BF 3对硅氧烷键的裂解。当使用足够过量的BF 3时,可以通过使用伪一级速率定律评估反应。发现相对于BF 3的顺序为4.7。所得到的速率常数可以被关联到σ * -值与ρ * -1.0 -值。确认了先前通过29 Si NMR光谱法测得的Me 3 SiO基为0.35的σ *值。
-
Siloxane basicity toward strong acid in nonpolar solution作者:Brian D. ShepherdDOI:10.1021/ja00015a010日期:1991.7The relative basicities of ten siloxanes and an ether were studied in benzene by determining from visible spectroscopic measurements the thermodynamic constants for the competition between the substrate and a reference base (4-chloro-2-nitroaniline) for acid (trifluoromethanesulfonic acid): RB•HA+S⇇RB+S•HA. The K eq values were considered as a measure of basicity with the following order established
-
一种制备甲基三(三甲基硅氧烷基)硅烷的方法
表征谱图
-
氢谱1HNMR
-
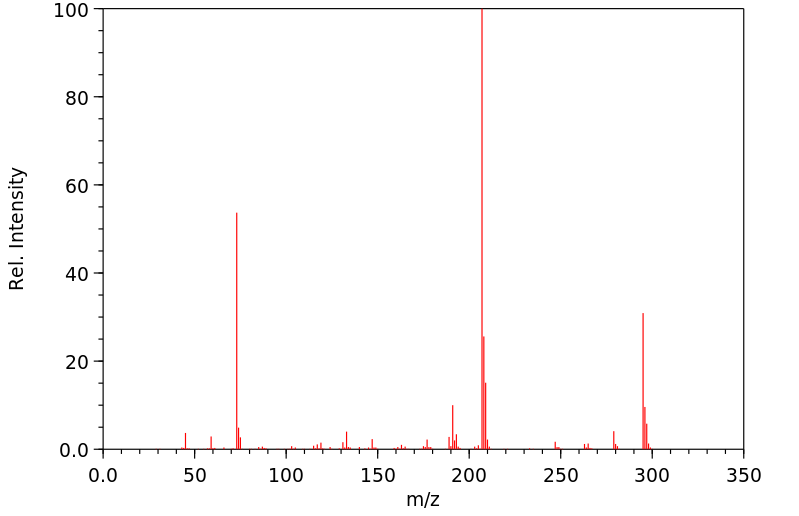
质谱MS
-
碳谱13CNMR
-
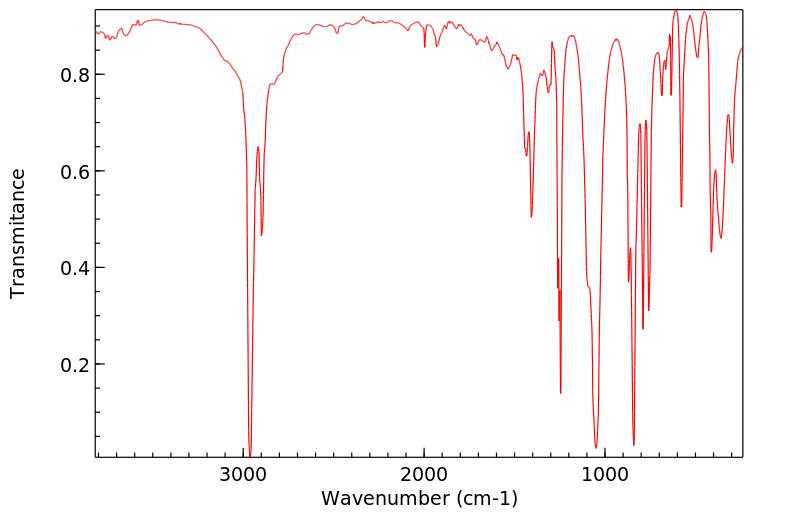
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷