4,5-二氯藜芦醚 | 2772-46-5
中文名称
4,5-二氯藜芦醚
中文别名
——
英文名称
4,5-dichloroveratrole
英文别名
4,5-dichloro-1,2-dimethoxybenzene;1,2-dichloro-4,5-dimethoxybenzene
CAS
2772-46-5
化学式
C8H8Cl2O2
mdl
——
分子量
207.056
InChiKey
RJYXLZQZBLGBOM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:83°C
-
沸点:297.35°C (rough estimate)
-
密度:1.3354 (rough estimate)
-
溶解度:氯仿(微溶)、甲醇(微溶、加热)
-
保留指数:1497;1483;1484;1484;1503
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2909309090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4,5-Dichloroveratrole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4,5-Dichloroveratrole
CAS number: 2772-46-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8Cl2O2
Molecular weight: 207.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4,5-Dichloroveratrole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4,5-Dichloroveratrole
CAS number: 2772-46-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8Cl2O2
Molecular weight: 207.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4,5-二氯愈创木酚 4,5-dichloroguaiacol 2460-49-3 C7H6Cl2O2 193.029 4,5-二氯儿茶酚 4,5-dichlorocatechol 3428-24-8 C6H4Cl2O2 179.003 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氯-1,2-二甲氧基苯 4-chloro-1,2-dimethoxybenzene 16766-27-1 C8H9ClO2 172.611 4,5-二氯儿茶酚 4,5-dichlorocatechol 3428-24-8 C6H4Cl2O2 179.003 —— 2,3-dichloro-5,6-dimethoxy-aniline 101252-15-7 C8H9Cl2NO2 222.071
反应信息
-
作为反应物:描述:参考文献:名称:Bushby, Richard J.; Hardy, Cristopher, Journal of the Chemical Society. Perkin transactions I, 1986, p. 721 - 724摘要:DOI:
-
作为产物:参考文献:名称:Halogenated volatiles from the fungus Geniculosporium and the actinomycete Streptomyces chartreusis摘要:在真菌Geniculosporium的头空间提取物中检测到了两种未经鉴定的氯化挥发性化合物X和Y。它们的质谱指向X为氯二甲氧基苯,Y为二氯二甲氧基苯的结构。X和Y的一些构造异构体的质谱已包含在我们的数据库中,证明它们非常相似,因此无法完全确定结构。为了明确结构,我们通过合成或从商业供应商处获得了X和Y的所有可能的构造异构体。质谱和气相色谱保留时间的比较严格确定了这两种氯化挥发性化合物的结构。氯化挥发性化合物并不是很常见,但溴化或甚至碘化的挥发性化合物更为罕见。令人惊讶的是,从Streptomyces chartreusis的头空间提取物中发现了甲基2-碘苯甲酸酯,这是一种新的天然产物,丰富了碘化天然产物家族。DOI:10.3762/bjoc.9.311
文献信息
-
Facile Synthesis of Chloro-substituted Aromatic Ethers by Use of Benzyltrimethylammonium Tetrachloroiodate作者:Shoji Kajigaeshi、Youichi Shinmasu、Shizuo Fujisaki、Takaaki KakinamiDOI:10.1246/cl.1989.415日期:1989.3The reaction of aromatic ethers with a calculated amount of benzyltrimethylammonium tetrachloroiodate in acetic acid (or dichloromethane) under mild conditions gave, selectively, the objective chloro-substituted aromatic ethers in good yields.
-
Novel Terphenyls as Selective Cyclooxygenase-2 Inhibitors and Orally Active Anti-inflammatory Agents作者:James J. Li、Monica B. Norton、Emily J. Reinhard、Gary D. Anderson、Susan A. Gregory、Peter C. Isakson、Carol M. Koboldt、Jaime L. Masferrer、William E. Perkins、Karen Seibert、Yan Zhang、Ben S. Zweifel、David B. ReitzDOI:10.1021/jm950878e日期:1996.1.1The sulfonamide analogs 17 and 21 were found to be much more potent COX-2 inhibitors and orally active anti-inflammatory agents than the corresponding methyl sulfone analogs 16 and 20, respectively, albeit with some decrease in COX-2 selectivity. Structure-activity relationship studies have determined that incorporation of two fluorine atoms in the central phenyl group, as in 20 and 21, is extremely一系列新的三联苯甲基砜和磺酰胺已被证明是高效的选择性环氧合酶2(COX-2)抑制剂。发现磺酰胺类似物17和21分别比相应的甲基砜类似物16和20更有效的COX-2抑制剂和口服活性抗炎药,尽管COX-2选择性有所降低。结构-活性关系研究已经确定,在中央苯基中引入两个氟原子(如20和21)对于体外COX-2的效力和选择性以及体内活性都极为有利。1,2-二芳基-4,5-二氟苯磺酰胺系列中几个值得注意的例子是21a-c,k,l,n(COX-2,IC50 = 0.002-0.004 microM),其中所有都具有体外COX-1 / COX-2选择性>1000。此外,在炎症的气袋模型中,磺酰胺21a,b,d,g,j,m,n,q显示出大大增强的口服活性,并抑制了90%以上的前列腺素E2产生。此外,在大鼠佐剂诱导的关节炎模型(ED50 = 0.05 mg / kg)和角叉菜胶诱导的痛觉过敏试验(ED50 =
-
Convenient One-Pot Synthesis of 9H-Carbazoles by Microwave Irradiation Employing a Green Palladium-Based Nanocatalyst作者:Darío C. Gerbino、H. Sebastián Steingruber、Pamela Mendioroz、María A. VolpeDOI:10.1055/s-0037-1610778日期:2021.11palladium-catalyzed tandem reaction for the one-pot synthesis of 9H-carbazoles under microwave irradiation is developed. This approach involves a sequential Buchwald–Hartwig amination and a direct arylation from affordable and inexpensive anilines and 1,2-dihaloarenes. For the development of this purpose, a novel and magnetically recoverable palladium nanocatalyst supported on a green biochar under ligand-free
-
13C and17O NMR study of methoxy groups in chlorinated Di- and trimethoxybenzenes作者:J. Knuutinen、E. KolehmainenDOI:10.1002/mrc.1260280407日期:1990.4and 1,4‐dimethoxybenzenes, 1,2,3‐trimethoxybenzenes and most of their chlorinated derivatives and some related brominated compounds were measured for CDCl3 solutions. The 17O NMR chemical shifts show up to 60 ppm dispersion. Comparison between the compounds with and without adjacent chlorine atoms (2,6‐di‐ and 2,4,6‐tri‐substitution) also showed a clear methoxy carbon chemical shift change. The number
-
Substituted biphenyl compounds for the treatment of inflammation申请人:G.D. Searle & Co.公开号:US20020169206A1公开(公告)日:2002-11-14A class of substituted biphenyl compounds is described for use in treating inflammation and inflammation-related disorders. Compounds of particular interest are defined by Formula III: 1 wherein each of R 11 through R 13 is independently selected from hydrido, halo, lower alkoxy, lower haloalkyl, amino, lower alkylamino, lower dialkylamino, and lower haloalkoxy; or wherein R 11 and R 12 together form —O(CH 2 ) n O—; wherein n is 1-2, inclusive; or a pharmaceutically-acceptable salt thereof.
表征谱图
-
氢谱1HNMR
-
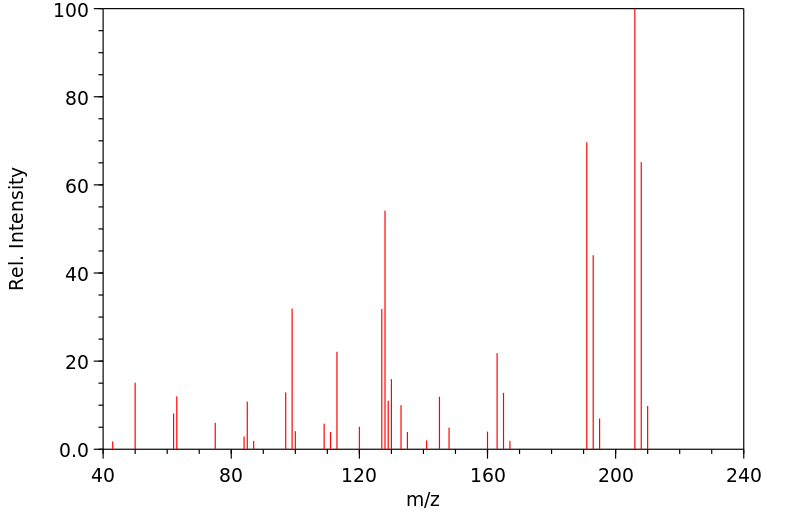
质谱MS
-
碳谱13CNMR
-
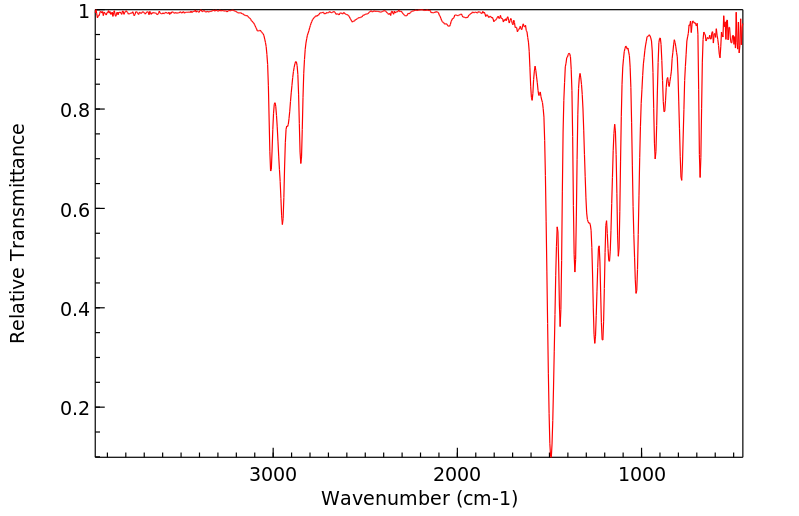
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫