2-甲基-1,3-恶噻戊环 | 17642-74-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:807;822;829;822
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:6
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:34.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Zorin, V. V.; Batyrbaev, N. A.; Zlot-skii, S. S., Journal of Organic Chemistry USSR (English Translation), 1984, vol. 20, p. 347 - 352摘要:DOI:
-
作为产物:描述:参考文献:名称:GERMASH, A. V.;ZORIN, V. V.;NIKOLAEVA, S. V.;ZLOTSKIJ, S. S.;TERENTEV, A.+, ZH. OBSHCH. XIMII, 1982, 52, N 10, 2328-2332摘要:DOI:
-
作为试剂:描述:2-异丙基-1,3-恶噻戊环 在 2-甲基-1,3-恶噻戊环 、 二叔丁基过氧化物 作用下, 以 氯苯 为溶剂, 130.0 ℃ 、100.0 kPa 条件下, 生成 硫代乙酸S-乙酯 、 ethyl β-methylthiopropionate参考文献:名称:Trifonova, V. N.; Zorin, V. V.; Batyrbaev, N. A., Journal of Organic Chemistry USSR (English Translation), 1986, vol. 22, # 7, p. 1386 - 1389摘要:DOI:
文献信息
-
Six membered heterocyclic oxathio compounds申请人:International Flavors & Fragrances Inc.公开号:US04042601A1公开(公告)日:1977-08-16One or more five or six membered oxathio heterocyclic compounds having one sulfur atom and one oxygen atom, such as an oxathiolane or an oxathiane, is used to alter, modify or enhance the flavor or aroma of a foodstuff, chewing gum or medicinal product. Among said oxathio heterocyclic compounds are the seven novel compounds: 2,6-dimethyl-2-phenyl-1,3-oxathiane 2-isobutyl-6-methyl-1,3-oxathiane Ethyl-2,6-dimethyl-1,3-oxathiane-2-acetate 2,6-dimethyl-2-acetyl-1,3-oxathiane 2-n-nonyl-6-methyl-1,3-oxathiane 2-isobutyl-1,3-oxathiolane 7-methyl(6-oxa-10-thiaspiro) 4.5-decane
-
An Effective Synthesis of 1,3-Oxathiolanes作者:Ludvík Streinz、Bohumír Koutek、David ŠamanDOI:10.1135/cccc19970665日期:——
2-Alkyl- or 2,2-dialkyl-1,3-oxathiolanes can be effectively prepared from aldehydes or ketones and 2-mercaptoethanol, with triisopropylsilyl triflate as a catalyst. The reaction is over within minutes and, despite the fact that water is nor removed during the reaction, the yields of products are high.
-
PREPARATION AND INFRARED SPECTRA OF DIVINYL SULPHIDE, 2-METHYL-1,3-THIOXOLANE, AND 1,4-THIOXANE作者:K. K. Georgieff、A. DupréDOI:10.1139/v59-159日期:1959.6.1
Dehydration of thiodiglycol at 195°–230° with potassium hydroxide produced a 36% yield of divinyl sulphide, 9–10% of 2-methyl-1,3-thioxolane, 7–8% of 1,4-thioxane, and 3.5% of vinyl 2-hydroxyethyl sulphide. Physical properties and infrared spectra are reported. Divinyl sulphide of high purity for polymer work was obtained by distillation in a Podbielniak column. Precautions that must be observed in the preparation, distillation, and storage are given.
-
Synthesis and Degradation of Vinyl Polymers with Evenly Distributed Thioacetal Bonds in Main Chains: Cationic DT Copolymerization of Vinyl Ethers and Cyclic Thioacetals作者:Mineto Uchiyama、Yukihiro Murakami、Kotaro Satoh、Masami KamigaitoDOI:10.1002/anie.202215021日期:2023.1.23were synthesized with periodically arranged in-chain thioacetal bonds via cationic degenerative chain-transfer copolymerization of vinyl ethers with a 7-membered cyclic thioacetal. The copolymers can be degraded into low- and controlled-molecular-weight polymers via hydrolysis. One-pot synthesis of multiblock copolymers and their degradation into diblock copolymers is also achieved.
-
Organic Sulfur Compounds; II<sup>1</sup>. Sulfur Dioxide as Catalyst in the Synthesis of Thioacetals from Aldehydes or Ketones and Alkanethiols, Alkanedithiols, or Hydroxyalkanethiols作者:Bogdan Burczyk、Zbigniew KortylewiczDOI:10.1055/s-1982-29960日期:——
表征谱图
-
氢谱1HNMR
-
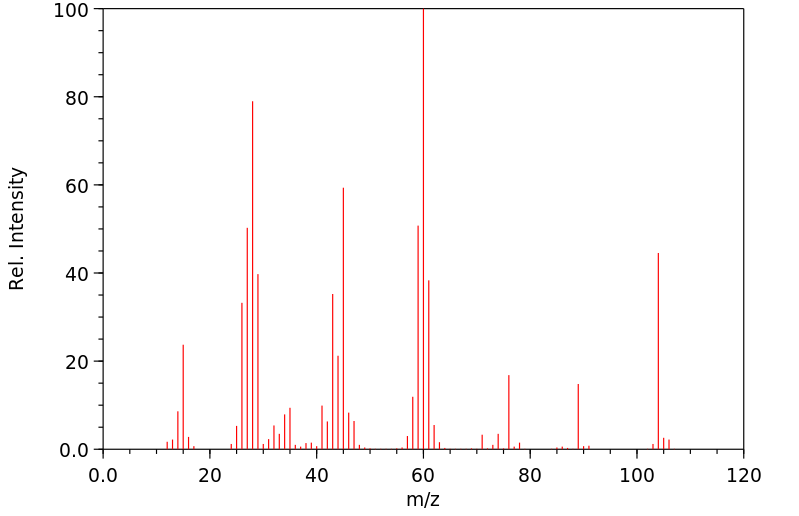
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息