3,3',4,4'-四氢-1,1'-联萘 | 5405-96-9
中文名称
3,3',4,4'-四氢-1,1'-联萘
中文别名
——
英文名称
3,3',4,4'-tetrahydro-1,1'-binaphthalene
英文别名
1,2-dihydro-4-(1,2-dihydronaphthalen-4-yl)naphthalene;3,3',4,4-tetrahydro-1,1'-binaphthyl;bisdialine;3,4,3',4'-Tetrahydro-[1,1']binaphthyl;1,1'-Binaphthyl, 3,3',4,4'-tetrahydro-;4-(3,4-dihydronaphthalen-1-yl)-1,2-dihydronaphthalene
CAS
5405-96-9
化学式
C20H18
mdl
——
分子量
258.363
InChiKey
QZIBLSYKUOHZFG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140 °C
-
沸点:401.7±35.0 °C(Predicted)
-
密度:1.129±0.06 g/cm3(Predicted)
-
保留指数:2432
计算性质
-
辛醇/水分配系数(LogP):4.8
-
重原子数:20
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,1',2,2',3,4-Hexahydro-1,1'-binaphthyl 193816-75-0 C20H20 260.379
反应信息
-
作为反应物:描述:参考文献:名称:中间体在3,3',4,4'-四氢-1,1'-联萘的脱氢中。一些氢化的1,1'-联萘和苯并[ j ]氟蒽摘要:3,3′,4,4′-四氢-1,1′-联萘可同时进行脱氢和环脱氢。已经制备并表征了具有不同氢化度的可能的中间体。这些中的一些已经被认为是中间体,因此已经追踪了两个平行反应的各自过程。DOI:10.1039/j39680002328
-
作为产物:描述:参考文献:名称:氯三甲基硅烷和锌对芳基和α,β-不饱和羰基化合物进行还原性脱氧的观察摘要:某些芳基和α,β-不饱和羰基化合物的还原McMurry型二羰基偶合生成烯烃可以使用三甲基氯硅烷和锌通过一种新颖的机理实现,该机理不涉及Pinacolic偶合和二醇脱氧。DOI:10.1039/c39860001803
文献信息
-
Oxokohlenstoffe und verwandte Verbindungen; 21. Mitteilung.<sup>1</sup>Diels-Alder Reaktion von 3-Halogeno-3-cyclobuten-1,2-dionen mit 1,1′-Bi-1-cycloalkenylen: Synthese dicycloalkano-anellierter Dihydrobenzocyclobutendione und Benzocyclobutendione作者:Arthur H. Schmidt、Christian Künz、Marianne Malmbak、Jörg ZyllaDOI:10.1055/s-1994-25490日期:——Oxocarbons and Related Compounds; Part 21.1 Diels-Alder Reactions of 3-Halogeno-3-cyclobutene-1,2-diones with 1,1′-Bi-1-cycloalkenyls. Synthesis of Dicycloalkano-annulated Dihydrobenzocyclobutenediones and Benzocyclobutenediones On controlled heating to 100°C, 3-chloro-3-cyclobutene-1,2-diones (5a) reacted with 1,1′-bi-1-cyclohexenyl (6) and 1, 1′-bi-1-cyclopentenyl (13) to give the dicycloalkano-annulated dihydrobenzocyclobutenediones 7 and 16, respectively. Treatment of carbon tetrachloride solutions of 7 and 16 with bromine at reflux temperature afforded the corresponding benzocyclobutenediones 9 and 17. Surprisingly, the reaction of 5a with 1,1′-bi-1-cycloheptenyl (14) and 1,1′-bi-1-cyclooctenyl (15) gave directly the benzocyclobutenediones 20 and 21. The hitherto unknown 3-bromo-3-cyclobutene-1,2-dione (5c) was prepared by treatment of 3-hydroxy-3-cyclobutene-1,2-dione (5b) with oxalic dibromide in 76% yield. Its reaction with the dienes 6, 13, 14 has been investigated.氧碳化合物及相关化合物;第21.1部分 Diels-Alder反应的3-卤代-3-环丁烯-1,2-二酮与1,1'-双-1-环烷烯的反应。二环烷基并环二氢苯并环丁二烯二酮和苯并环丁二烯二酮的合成 在100°C下加热控制,3-氯-3-环丁烯-1,2-二酮(5a)与1,1'-双-1-环己烯(6)和1,1'-双-1-环戊烯(13)反应,分别得到二环烷基并环二氢苯并环丁二烯二酮7和16。将7和16的四氯化碳溶液在回流温度下与溴反应,分别得到相应的苯并环丁二烯二酮9和17。令人惊讶的是,5a与1,1'-双-1-环庚烯(14)和1,1'-双-1-环辛烯(15)的反应直接得到苯并环丁二烯二酮20和21。迄今未知的3-溴-3-环丁烯-1,2-二酮(5c)通过3-羟基-3-环丁烯-1,2-二酮(5b)与草酸二溴反应制备,产率为76%。其与二烯6、13、14的反应已进行了研究。
-
Multimetallic Ni- and Pd-Catalyzed Cross-Electrophile Coupling To Form Highly Substituted 1,3-Dienes作者:Astrid M. Olivares、Daniel J. WeixDOI:10.1021/jacs.7b13601日期:2018.2.21synthesis of highly substituted 1,3-dienes from the coupling of vinyl bromides with vinyl triflates is reported for the first time. The coupling is catalyzed by a combination of (5,5'-bis(trifluoromethyl)-2,2'-bipyridine)NiBr2 and (1,3-bis(diphenylphosphino)propane)PdCl2 in the presence of a zinc reductant. This method affords tetra- and penta-substituted 1,3-dienes that would otherwise be difficult
-
Ligand-Free Iron-Catalyzed Carbon (sp<sup>2</sup>)–Carbon (sp<sup>2</sup>) Oxidative Homo-Coupling of Alkenyllithiums作者:Zhuliang Zhong、Zhi-Yong Wang、Shao-Fei Ni、Li Dang、Hung Kay Lee、Xiao-Shui Peng、Henry N. C. WongDOI:10.1021/acs.orglett.8b03893日期:2019.2.1and hexa-substituted 1,3-butadienes. This one-pot procedure involves lithium–iodine exchange to generate the corresponding vinyllithium intermediates. A subsequent iron-catalyzed ligand-free oxidative homo-coupling eventually led to the formation of 1,3-butadienes in acceptable to excellent isolated yields.
-
Synthesis of Highly Substituted Polyenes by Palladium-Catalyzed Cross-Couplings of Sterically Encumbered Alkenyl Bromides and<i>N</i>-Tosylhydrazones作者:Miguel Paraja、Raquel Barroso、M. Paz Cabal、Carlos ValdésDOI:10.1002/adsc.201601155日期:2017.3.20bromides. The reaction proceeds efficiently when a combination of a highly substituted bromoalkene and a hydrazone derived from a ketone are employed, pointing to the convenience of a sterically encumbered environment. This unprecedented process allows for the stereocontrolled preparation of highly substituted dienes and polyenes.
-
Straightforward Synthesis of a Fluorous Tetraarylporphyrin: an Efficient and Recyclable Sensitizer for Photooxygenation Reactions作者:Gianluca Pozzi、Lászlo Mercs、Orsolya Holczknecht、Fabio Martimbianco、Fabrizio FabrisDOI:10.1002/adsc.200606092日期:2006.8A fluorous tetraarylporphyrin has been prepared in a single reaction step starting from commercially available 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin [TPP(OH)4]. The new compound was successfully employed as a sensitizer in photooxygenation reactions carried out under homogeneous conditions, showing activity comparable to the standard 5,10,15,20-tetraphenylporphyrin (TPP) sensitizer. Photooxygenation
表征谱图
-
氢谱1HNMR
-
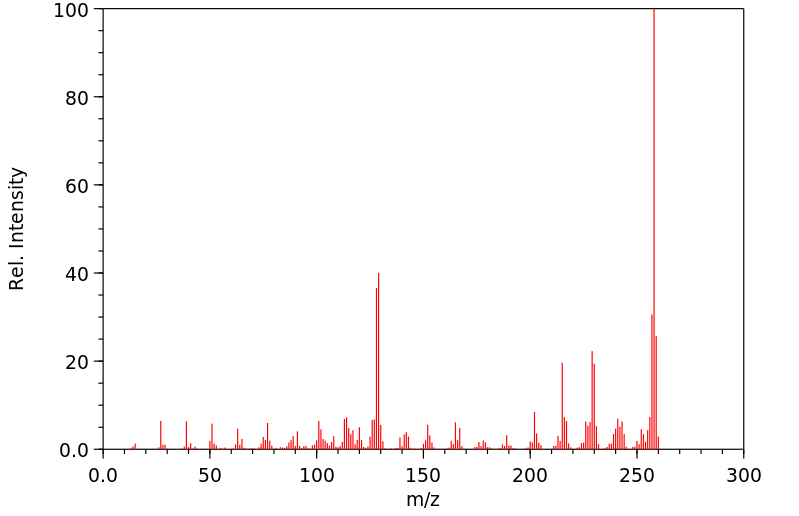
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮