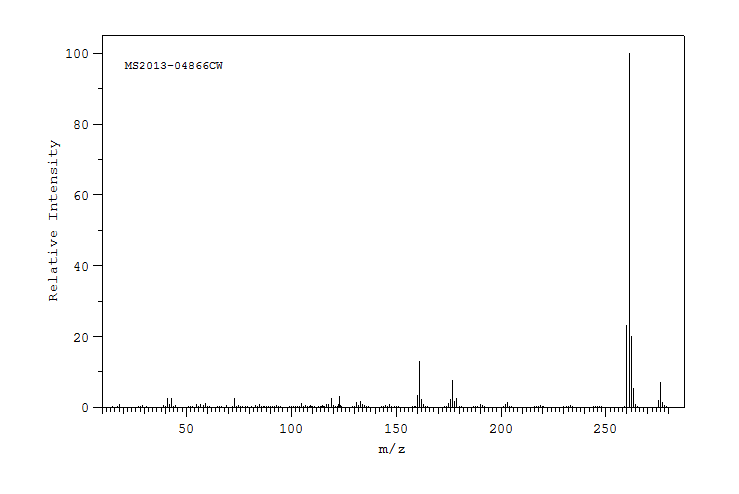4-三甲硅基苯硼酸频呐醇酯 | 1186026-67-4
中文名称
4-三甲硅基苯硼酸频呐醇酯
中文别名
4-三甲基甲硅烷基苯硼酸频那醇酯
英文名称
trimethyl(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)silane
英文别名
4-trimethylsilylphenylboronic acid pinacol ester;trimethyl-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]silane
CAS
1186026-67-4
化学式
C15H25BO2Si
mdl
——
分子量
276.259
InChiKey
OPGOPAKVZDQUMR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:335.4±25.0 °C(Predicted)
-
密度:0.95±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.53
-
重原子数:19
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存储条件:2-8℃,干燥且密封。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-Trimethylsilylphenylboronic acid pinacol ester
Synonyms: 4,4,5,5-Tetramethyl-2-(4-trimethylsilanyl-phenyl)-[1,3,2]dioxaborolane
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Trimethylsilylphenylboronic acid pinacol ester
CAS number: 1186026-67-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C15H25BO2Si
Molecular weight: 276.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-Trimethylsilylphenylboronic acid pinacol ester
Synonyms: 4,4,5,5-Tetramethyl-2-(4-trimethylsilanyl-phenyl)-[1,3,2]dioxaborolane
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Trimethylsilylphenylboronic acid pinacol ester
CAS number: 1186026-67-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C15H25BO2Si
Molecular weight: 276.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-溴苯硼酸频那醇酯 p-bromophenylboronic acid pinacol ester 68716-49-4 C12H16BBrO2 282.973 4-(4,4,5,5-四甲基-1,3,2-二氧杂硼烷-2-基)氯苯 4-chlorophenylboronic acid pinacol ester 195062-61-4 C12H16BClO2 238.522 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 苯硼酸频哪醇酯 2-phenyl-4,4,5,5-tetramethyl-1,3,2-dioxoborole 24388-23-6 C12H17BO2 204.077 4,4'-二(4,4,5,5-四甲基-1,3,2-二氧硼戊环-2-基)联苯 4,4'-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,1'-biphenyl 207611-87-8 C24H32B2O4 406.138 4-溴-4'-联苯硼酸频哪醇酯 2-(4'-bromo-[1,1'-biphenyl]-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 1414997-84-4 C18H20BBrO2 359.071 —— 2-(4'-fluoro-[1,1'-biphenyl]-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 1359844-10-2 C18H20BFO2 298.165 2-(4'-氯-二苯基-4-基)-4,4,5,5-四甲基-[1,3,2]二恶硼烷 2-(4'-chloro-[1,1'-biphenyl]-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 942589-53-9 C18H20BClO2 314.62 —— 2-(4'-(tert-butyl)-[1,1'-biphenyl]-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane —— C22H29BO2 336.282 —— 1-(4'-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1'-biphenyl]-4-yl)ethan-1-one 269410-19-7 C20H23BO3 322.212
反应信息
-
作为反应物:描述:4-三甲硅基苯硼酸频呐醇酯 在 tetrakis(acetonitrile)palladium(II) bis(tetrafluoroborate) 、 邻四氯苯醌 作用下, 以 氯仿 、 1,2-二氯乙烷 为溶剂, 反应 10.0h, 以70%的产率得到4,4'-二(4,4,5,5-四甲基-1,3,2-二氧硼戊环-2-基)联苯参考文献:名称:Pd/邻氯苯醌催化芳基三甲基硅烷的氧化均偶联反应摘要:Pd/o-氯苯醌催化体系实现了芳基三甲基硅烷的实际氧化均偶联反应。该反应显示出对溴、氟、酯和甲氧基的良好官能团耐受性,得到一系列带有吸电子和供电子基团的联芳基。硼酸酯基团也保留在联芳基上,没有任何 Ar-B 键断裂,这对于正交耦合非常有利。DOI:10.1246/cl.170723
-
作为产物:描述:对-(三甲基甲硅烷)苯硫酚 在 [1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride 、 sodium hydroxide 、 lithium hexamethyldisilazane 作用下, 以 四氢呋喃 、 乙醇 为溶剂, 反应 18.5h, 生成 4-三甲硅基苯硼酸频呐醇酯参考文献:名称:钯催化本位芳基硫化物与Diborons的-Borylation摘要:一种催化宫浦型本位与二硼试剂芳基硫化物的-borylation已经实现,从而提供合成的使用arylboronate酯。将本来就不愿意的C–S键转换为C–B键的关键条件包括钯-NHC(N-杂环卡宾)预催化剂,双(频哪醇)二硼和六甲基二硅叠氮化锂。该协议适用于合理范围的芳基烷基硫化物。在二苯硫醚的反应中观察到双重硼化。DOI:10.1021/acs.orglett.6b01305
文献信息
-
Selective Transformation of Strychnine and 1,2-Disubstituted Benzenes by C–H Borylation作者:Yutaro Saito、Kotono Yamanoue、Yasutomo Segawa、Kenichiro ItamiDOI:10.1016/j.chempr.2020.02.004日期:2020.4as natural products, pharmaceuticals, and π-conjugated systems are at the heart of constructing and modifying organic molecules, whereby the selectivity and predictability are of the utmost importance. Herein, we report the highly C3-selective C–H borylation of strychnine along with olefin isomerization, catalyzed by an iridium complex with a diphosphine ligand. This method enabled us to rapidly produce
-
Identification and structure activity relationship of novel flavone derivatives that inhibit the production of nitric oxide and PGE 2 in LPS-induced RAW 264.7 cells作者:Ji-Young An、Hwi-Ho Lee、Ji-Sun Shin、Hyung-Seok Yoo、Jong Seon Park、Seung Hwan Son、Sang Won Kim、Jihyun Yu、Jun Lee、Kyung-Tae Lee、Nam-Jung KimDOI:10.1016/j.bmcl.2017.03.057日期:2017.6In an effort to identify novel anti-inflammatory compounds, a series of flavone derivatives were synthesized and biologically evaluated for their inhibitory effects on the production of nitric oxide (NO) and prostaglandin E2 (PGE2), representative pro-inflammatory mediators, in LPS-induced RAW 264.7 cells. Their structure-activity relationship was also investigated. In particular, we found that compound
-
Direct Stereospecific Amination of Alkyl and Aryl Pinacol Boronates作者:Scott N. Mlynarski、Alexander S. Karns、James P. MorkenDOI:10.1021/ja305448w日期:2012.10.10The direct amination of alkyl and aryl pinacol boronates is accomplished with lithiated methoxyamine. This reaction directly provides aliphatic and aromatic amines, stereospecifically, and without preactivation of the boronate substrate.
-
Cross‐Coupling through Ag(I)/Ag(III) Redox Manifold作者:Luca Demonti、Nathalie Saffon‐Merceron、Nicolas Mézailles、Noel NebraDOI:10.1002/chem.202102836日期:2021.11.5trifluoromethylation via AgI/AgIII redox shuttles are herein deciphered. This innovative cross-coupling, analogous to the CEL coupling popularized by copper, involves: i) easy AgI/AgIII 2e− oxidation; ii) phen ligation to AgIII; iii) boron-to-AgIII aryl transfer; and iv) benzotrifluoride production from aryl-AgIII intermediates. Now, AgIIICF3 chemistry is safe and reachable from AgF, KF, CF3TMS and air.
-
Sodium silylsilanolate enables nickel-catalysed silylation of aryl chlorides作者:Kenshiro Hitoshio、Hiroki Yamagishi、Jun Shimokawa、Hideki YorimitsuDOI:10.1039/d1cc02683f日期:——Structurally diverse aryl chlorides were silylated with sodium silylsilanolate reagents in the presence of a Ni(cod)2 catalyst complexed with a phosphine ligand; PMe2Ph for electron-rich substrates, and PCy2Ph for electron-deficient ones. The mild reaction conditions allowed the silylation of various aryl chlorides including functionalised drug molecules.
表征谱图
-
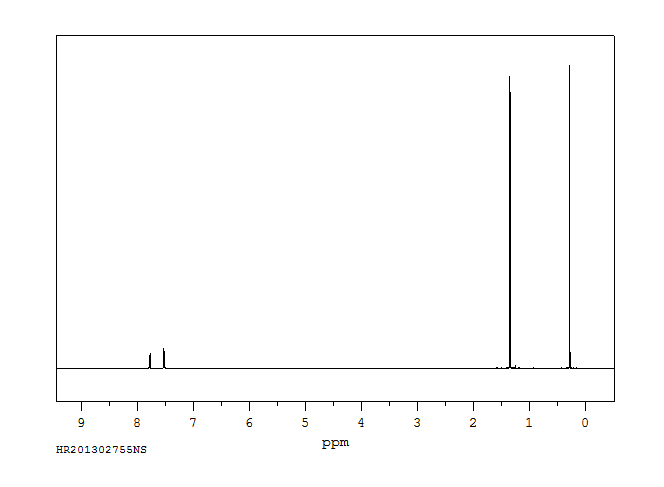
氢谱1HNMR
-
质谱MS
-
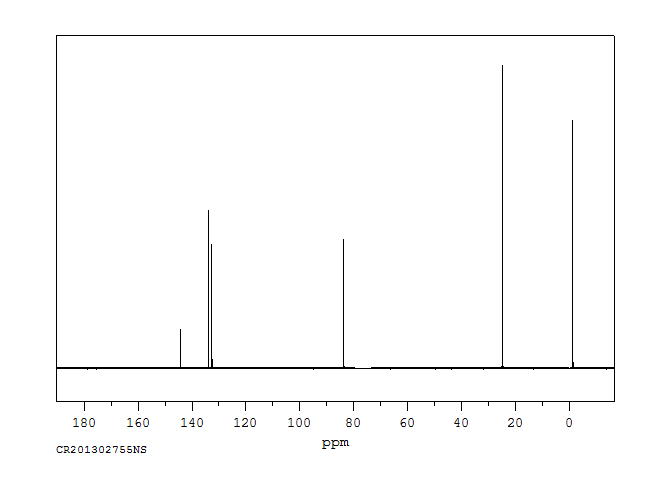
碳谱13CNMR
-
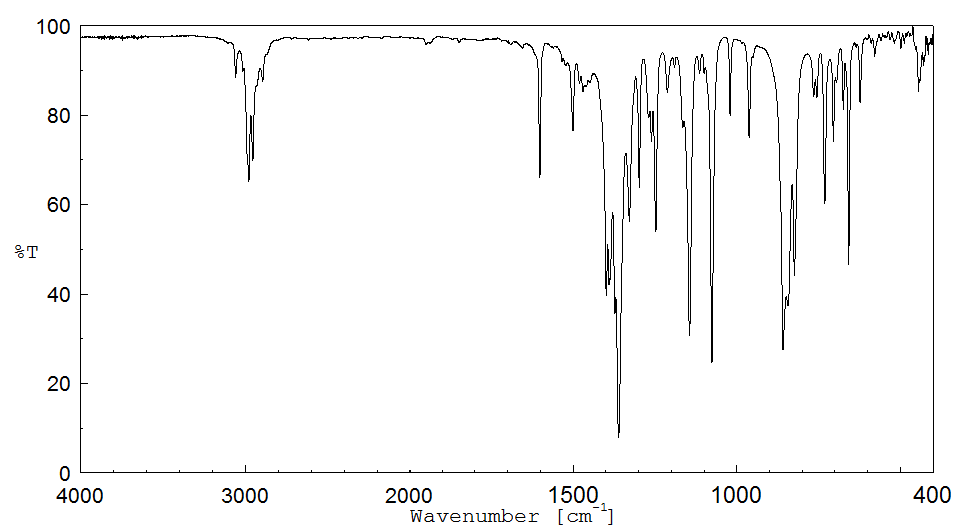
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷