3-氯-Alpha,Alpha-二溴甲苯 | 70288-97-0
中文名称
3-氯-Alpha,Alpha-二溴甲苯
中文别名
3-氯-α,α-二溴甲苯
英文名称
m-chlorobenzal bromide
英文别名
1-chloro-3-(dibromomethyl)benzene;3-Chlorobenzal bromide
CAS
70288-97-0
化学式
C7H5Br2Cl
mdl
——
分子量
284.378
InChiKey
GXTJYNIKOLTVNC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R20/21/22,R34
-
海关编码:2903999090
-
储存条件:存于阴凉干燥处
SDS
| Name: | 3-Chlorobenzal bromide 98% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 70288-97-0 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 70288-97-0 | 3-Chlorobenzal bromide | 98% | 274-538-1 |
Risk Phrases: 20/21 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation and in contact with skin. Causes burns.
Potential Health Effects
Eye:
Causes eye burns. Lachrymator (substance which increases the flow of tears).
Skin:
Harmful if absorbed through the skin. Causes skin burns.
Ingestion:
Causes gastrointestinal tract burns.
Inhalation:
Harmful if inhaled. Causes chemical burns to the respiratory tract.
Chronic:
Chronic exposure may cause effects similar to those of acute exposure.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid immediately.
Skin:
In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Get medical aid immediately. Wash clothing before reuse.
Ingestion:
If swallowed, do NOT induce vomiting. Get medical aid immediately.
If victim is fully conscious, give a cupful of water. Never give anything by mouth to an unconscious person.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Do not ingest or inhale. Use only in a chemical fume hood. Discard contaminated shoes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 70288-97-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear colorless to yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H5Br2Cl
Molecular Weight: 284.38
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at normal temperatures in tightly closed containers under an inert atmosphere.
Conditions to Avoid:
Incompatible materials, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 70288-97-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Chlorobenzal bromide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: Pseudomonas putida:
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S
Hazard Class: 8
UN Number: 3265
Packing Group: III
IMO
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S
Hazard Class: 8
UN Number: 3265
Packing Group: III
RID/ADR
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S
Hazard Class: 8
UN Number: 3265
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 20/21 Harmful by inhalation and in contact with
skin.
R 34 Causes burns.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 70288-97-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 70288-97-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 70288-97-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:3-氯-Alpha,Alpha-二溴甲苯 在 四氯化锡 、 sodium hydride 作用下, 以 N,N-二甲基甲酰胺 、 苯 为溶剂, 反应 9.0h, 生成 2-(3-Chloro-phenyl)-4,5-dimethyl-3,6-dihydro-2H-selenopyran参考文献:名称:氯化锡诱导的双(N,N-二甲基氨基甲酰硒)甲烷的不对称 CSe 键断裂:硒醛的新生成摘要:用 SnCl4 处理双(N,N-二甲基氨基甲酰硒)甲烷以中等至高产率获得 β-1,3,5-三硒烷,反应的关键中间体,即酰基硒离子和硒醛被成功捕获使用烯丙基三甲基硅烷或2,3-二甲基-1,3-丁二烯分别得到烯丙基化产物或环加合物。© 2006 Wiley Periodicals, Inc. 杂原子化学 17:125–135, 2006; 在线发表于 Wiley InterScience (www.interscience.wiley.com)。DOI 10.1002/hc.20190DOI:10.1002/hc.20190
-
作为产物:描述:参考文献:名称:Easy access for the synthesis of 2-aryl 2,3-dihydroquinazolin-4(1H)-ones using gem-dibromomethylarenes as synthetic aldehyde equivalent摘要:从
gem -二溴甲基芳烃和2-氨基苯甲酰胺一步合成2,3-二氢喹唑啉-4(1H )-酮。DOI:10.1039/c4ra02312a
文献信息
-
[EN] COMPOUNDS AND THEIR USE TO TREAT HISTAMINE H3 RELATED DISORDERS<br/>[FR] COMPOSÉS ET LEUR UTILISATION POUR LE TRAITEMENT DE TROUBLES ASSOCIÉS AU RÉCEPTEUR H3 DE L'HISTAMINE申请人:TAKEDA PHARMACEUTICAL公开号:WO2013027001A1公开(公告)日:2013-02-28The present invention provides compounds of formula (1) and pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, R20, m, n, p, q, Q1, Q2, Q3, Q4, Q5, Q6, X1, X2, X3, X4, A1 and L1, are as defined in the specification, processes for their preparation, pharmaceutical compositions containing them and their use in therapy.本发明提供了式(1)的化合物及其药学上可接受的盐,其中R1、R2、R3、R4、R5、R6、R7、R8、R9、R10、R11、R12、R13、R14、R15、R16、R17、R18、R19、R20、m、n、p、q、Q1、Q2、Q3、Q4、Q5、Q6、X1、X2、X3、X4、A1和L1如规范中所定义,其制备方法,含有它们的药物组合物以及它们在治疗中的用途。
-
[EN] HETEROARYL COMPOUNDS USEFUL AS INHIBITORS OF SUMO ACTIVATING ENZYME<br/>[FR] COMPOSÉS HÉTÉROARYLE UTILES EN TANT QU'INHIBITEURS DE L'ENZYME D'ACTIVATION SUMO申请人:DUFFEY MATTHEW O公开号:WO2016004136A1公开(公告)日:2016-01-07Disclosed are chemical entities which are compounds of formula (I); or pharmaceutically acceptable salts thereof; wherein Y, Ra, Ra', Rb, Rc, X1, X2, X3, Rd, Z1, and Z2 have the values described herein and stereochemical configurations depicted at asterisked positions indicate absolute stereochemistry. Chemical entities according to the disclosure can be useful as inhibitors of Sumo Activating Enzyme (SAE). Further provided are pharmaceutical compositions comprising a compound of the disclosure and methods of using the compositions in the treatment of proliferative, inflammatory, cardiovascular, and neurodegenerative diseases or disorders.
-
一种苄基溴的制备方法申请人:浙江师范大学公开号:CN107098791B公开(公告)日:2021-01-26
-
Visible-Light-Driven Oxidative Mono- and Dibromination of Benzylic sp3 C–H Bonds with Potassium Bromide/Oxone at Room Temperature作者:Wenjun Lu、Mengdi Zhao、Meiqi LiDOI:10.1055/s-0037-1610651日期:2018.12to achieving both high yield and high selectivity in these brominations. Mono- and difluorides can be conveniently prepared through nucleophilic substitutions of the benzylic bromides with potassium fluoride. Benzylic sp3 C–H bonds have been successfully brominated with potassium bromide by using Oxone as an oxidant in water/dichloromethane under visible light at room temperature. Toluene, ethylbenzene◊ MZ和ML同等贡献这项工作。 抽象的 在室温下可见光下,在水/二氯甲烷中使用Oxone作为氧化剂,已成功地用溴化钾溴化了苯甲酸sp 3 C–H键。带有吸电子基团的甲苯,乙苯和其他烷基苯,例如Br,Cl,COMe,CO 2 Et,CO 2 H,CN或NO 2在该反应中,以良好或优异的产率提供相应的苄基一溴化物。在过量的溴化钾存在下,也可以在延长的反应时间内生成二溴化物。控制可见光(〜500 lux)的照度对于实现这些溴化物的高产率和高选择性至关重要。一氟化物和二氟化物可以通过用氟化钾对苄基溴化物进行亲核取代而方便地制备。 在室温下可见光下,在水/二氯甲烷中使用Oxone作为氧化剂,已成功地用溴化钾溴化了苯甲酸sp 3 C–H键。带有吸电子基团的甲苯,乙苯和其他烷基苯,例如Br,Cl,COMe,CO 2 Et,CO 2 H,CN或NO 2在该反应中,以良好或优异的产率提供相应的苄基
-
High selectively oxidative bromination of toluene derivatives by the H2O2–HBr system作者:Jie Ju、Yu Jin Li、Jian Rong Gao、Jian Hong Jia、Liang Han、Wei Jian Sheng、Yi Xia JiaDOI:10.1016/j.cclet.2010.11.005日期:2011.4hydrogen bromide illuminated by a 60 W incandescent light bulb serves as a source of bromine radicals. Various substituted toluenes (NO2, Cl, Br, H, CH3) were high selectively brominated at the benzyl position for monobromination in CH2Cl2 at ice water with catalyst free. This simple but effective bromination of toluene derivatives with an aqueous H2O2–HBr system is characterized with the use of inexpensive
表征谱图
-
氢谱1HNMR
-
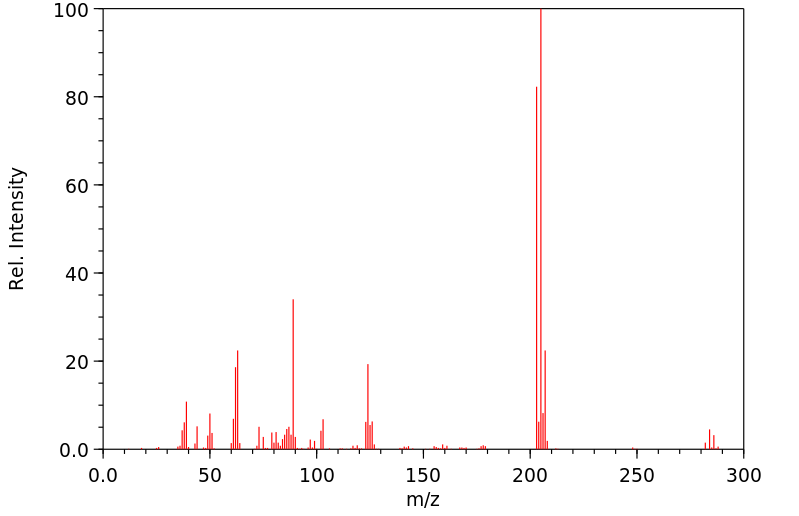
质谱MS
-
碳谱13CNMR
-
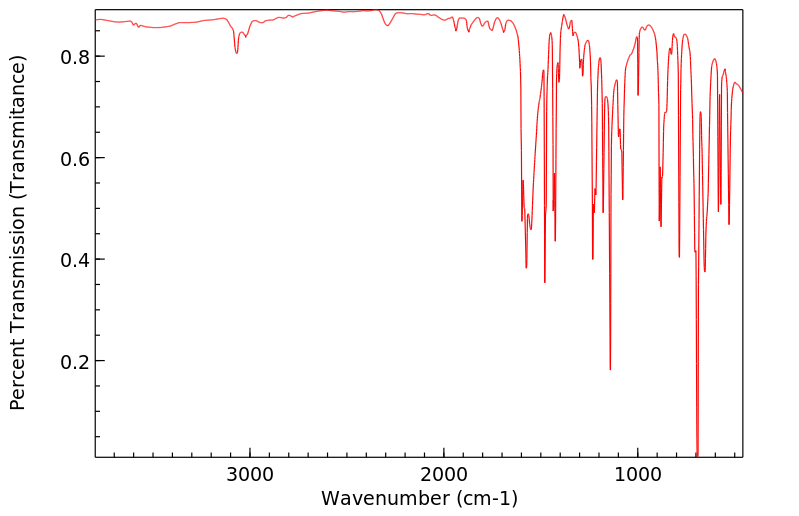
红外IR
-
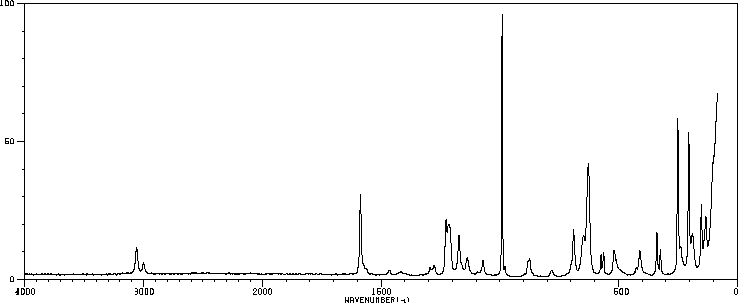
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫