2-三氯甲基-1,3-二氧戊环 | 5660-66-2
中文名称
2-三氯甲基-1,3-二氧戊环
中文别名
——
英文名称
2-(trichloromethyl)-1,3-dioxolane
英文别名
——
CAS
5660-66-2
化学式
C4H5Cl3O2
mdl
MFCD06210141
分子量
191.441
InChiKey
HNPXJOYUUGWILX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2,2,2-trichloro-1-(2-hydroxy-ethoxy)-ethanol 53256-76-1 C4H7Cl3O3 209.457 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-(二氯甲基)-1,3-二氧戊环 2-dichloromethyl-[1,3]dioxolane 2612-35-3 C4H6Cl2O2 156.996
反应信息
-
作为反应物:描述:参考文献:名称:Radical addition of 2-trichloromethyl-1,3-dioxolane to vinyltrimethylsilane摘要:DOI:10.1007/bf00957550
-
作为产物:描述:参考文献:名称:Hibbert; Morazain; Paquet, Canadian Journal of Research, 1930, vol. 2, p. 139摘要:DOI:
文献信息
-
A New Synthetic Approach to α-Chlorocinnamaldehydes作者:Valentine Nenajdenko、Alexander Reznichenko、Olesya Lenkova、Alexey Shastin、Elizabeth BalenkovaDOI:10.1055/s-2004-837303日期:——A new simple and efficient transformation of aromatic aldehydes into α-chlorocinnamaldehydes is described. Catalytic olefination reaction of hydrazones of aromatic aldehydes with 2-trichloromethyl-1,3-dioxolane gives ethylene acetals of target alkenes in moderate to good yields. The reaction proceeds stereoselectively to form preferably Z-isomers.
-
2-DICHLOROMETHYL-1,3-DIOXOLAN-2-YL ORTHOESTERS. A POTENTIAL PROTECTING GROUP FOR SUGAR DERIVATIVES作者:H. Özgener、L. YüceerDOI:10.1081/car-120016854日期:——with acetal protected sugar derivatives in neutral media gave 2-dichloromethyl-1,3-dioxolan-2-yl orthoesters. These orthoesters are readily hydrolysed under mild acidic conditions offering a new method for the preparation of readily removable protecting groups. This strategy was realised in the preparation of 6-O-acetyl-3,5-di-O-methyl-1,2-O-isopropylidene-α-d-glucofuranose.
-
Asymmetric Organocatalyzed Friedel–Crafts Reaction of Trihaloacetaldehydes and Phenols作者:David Svestka、Jan Otevrel、Pavel BobalDOI:10.1002/adsc.202200180日期:2022.7.5Herein we report the asymmetric organocatalyzed method for the Friedel–Crafts reaction between activated phenols and trihaloacetaldehydes. A three-phase screening including 41 compounds was employed to identify a catalyst structure based on 3,5-dinitrobenzamide of 9-amino-epi-cinchonidine as the lead catalytic molecule. Under the optimized reaction conditions, the above catalyst offered trihalohydroxyalkylated
-
Meldrum; Vad, Journal of the Indian Chemical Society, 1936, vol. 13, p. 119作者:Meldrum、VadDOI:——日期:——
-
Ketene Acetals. XIX. 2-Methylene-1,3-dioxolanes and 1,3-Dioxanes作者:S. M. McElvain、Michael J. CurryDOI:10.1021/ja01191a071日期:1948.11
表征谱图
-
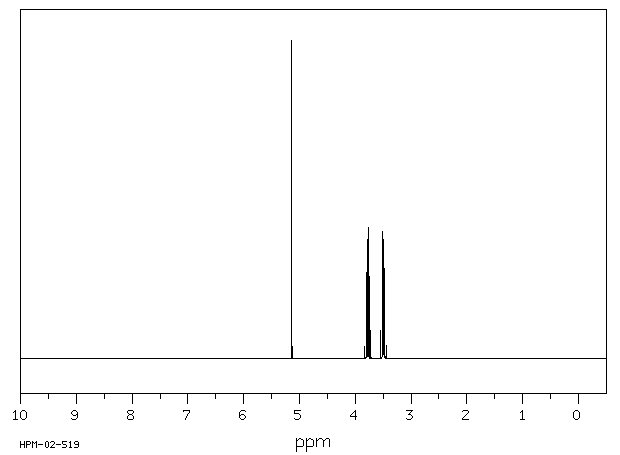
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-甲基-4-叔-丁基-1,3-二氧戊环
过氧竹红菌素
辛醛丙二醇缩醛
碘丙甘油
甜瓜醛丙二醇缩醛
甘油缩甲醛
甘油缩甲醛
环辛基甲醛乙烯缩醛
环戊二烯内过氧化物
环己丙胺,1-(1,3-二噁戊环-2-基)-
环丙羧酸,2-乙酰基-,甲基酯,(1R-顺)-(9CI)
氯乙醛缩乙二醇
柠檬醛乙二醇缩醛
异戊醛丙二醇缩醛
异丁醛-丙二醇缩醛
奥普碘铵
多米奥醇
多效缩醛
壬醛丙二醇缩醛
四吖戊啶,5-(1-吡咯烷基)-
亲和素
二氰苯乙烯酮乙烯缩醛
乙酮,1-(2-环辛烯-1-基)-,(-)-(9CI)
乙基1,3-二氧戊环-4-羧酸酯
丙炔醛乙二醇缩醛
三甲基-[(2-甲基-1,3-二氧戊环-4-基)甲基]铵碘化物
三氟乙烯臭氧化物
三丁基(1,3-二恶烷-2-基甲基)溴化鏻
[2-(2-碘乙基)-1,3-二氧戊环-4-基]甲醇
6,8-二氧杂二螺[2.1.4.2]十一烷
6,7-二氧杂双环[3.2.1]辛-2-烯-8-羧酸
5H,8H-呋喃并[3,4:1,5]环戊二烯并[1,2-d]-1,3-二噁唑(9CI)
5-过氧化氢基-5-甲基-1,2-二恶烷-3-酮
5-嘧啶羧酸,4-(2-呋喃基)-1,2,3,4-四氢-6-甲基-2-羰基-,1-甲基乙基酯
5-(哌嗪-1-基)苯并呋喃-2-甲酰胺
5-(1,3-二氧杂烷-2-基)呋喃-2-磺酰氯
5-(1,3-二氧戊环-2-基)戊腈
5,5-二羟基戊醛
4a-乙基-2,4a,5,6,7,7a-六氢-4-(3-羟基苯基)-1-甲基-1H-1-吡喃并英并啶
4-硝基-4-丙基辛醛乙烯缩醛
4-甲基-4-硝基辛醛乙烯缩醛
4-甲基-2-戊基-1,3-二氧戊环
4-甲基-2-十一烷基-1,3-二氧戊环
4-甲基-2-[(1E)-1-戊烯-1-基]-1,3-二氧戊环
4-甲基-2-(三氯甲基)-1,3-二氧戊环
4-甲基-2-(2-(甲硫基)乙基)-1,3-二氧戊环
4-甲基-2-(1-丙烯基)-1,3-二氧戊环
4-甲基-1,3-二氧戊环
4-烯丙基-4-甲基-2-乙烯基-1,3-二氧戊环
4-溴-3,5,5-三甲基二氧戊环-3-醇