2-吗啉基苯并噻唑 | 4225-26-7
中文名称
2-吗啉基苯并噻唑
中文别名
——
英文名称
4-(1,3-benzothiazol-2-yl)morpholine
英文别名
4-(benzo[d]thiazol-2-yl)morpholine;2-(4-morpholinyl)benzothiazole;2-Morpholino-benzthiazol;2-(morpholino)benzothiazole;2-(morpholin-4-yl)benzothiazole;2-morpholinobenzo[d]thiazole;2-(Morpholino)-benzothiazol
CAS
4225-26-7
化学式
C11H12N2OS
mdl
MFCD00054179
分子量
220.295
InChiKey
VVUVJGRVEYHIHC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112-113 °C
-
沸点:368.7±52.0 °C(Predicted)
-
密度:1.299±0.06 g/cm3(Predicted)
-
保留指数:1945
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:15
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:53.6
-
氢给体数:0
-
氢受体数:4
SDS
反应信息
-
作为反应物:描述:2-吗啉基苯并噻唑 在 2,6-二甲基吡啶 、 2,4,5,6-四(9H-咔唑-9-基)异酞腈 、 氧气 作用下, 以 乙腈 为溶剂, 反应 24.0h, 以45%的产率得到2-(N-(benzo[d]thiazol-2-yl)formamido)ethyl formate参考文献:名称:使用 4CzIPN 作为光催化剂的吗啉衍生物的可见光介导的有氧氧化 C(sp3)-C(sp3) 键裂解摘要:在此,开发了一种用于惰性 C( sp 3 )-C( sp 3 ) 键有氧氧化裂解的无金属策略。以可见光为能源,O 2为氧化剂,在温和条件下进行吗啉衍生物的解构。该程序证明了合适的选择性和官能团耐受性。此外,基于一系列机制探索和控制实验,提出了涉及激进过程的可能机制。DOI:10.1002/adsc.202100455
-
作为产物:描述:N-(2-fluorophenyl)morpholine-4-carbothioamide 在 sodium carbonate 、 copper(ll) bromide 作用下, 以 二甲基亚砜 为溶剂, 反应 22.0h, 以0.33 g的产率得到2-吗啉基苯并噻唑参考文献:名称:Cu(ii)催化的硫脲向硫代氨基胍/ 2 /氨基苯并噻唑的化学选择性氧化转化†摘要:具有催化量的Cu(II)盐的2-卤代芳基-仲-烷基不对称硫脲(Tu)(卤代= -F,-Cl)原位氧化成其二硫键中间体,然后亚胺-二硫键重排得到硫代氨基胍基(标签)在室温下的部分。在此过程中,Cu(II)还原为Cu(I),并与Tag部分形成复合物,在用Mg处理后,可从中分离Tag部分。氨。但是,当在高温下用催化量的Cu(II)盐进行相同的反应时,带有邻卤素的Tu (-F,-Cl)通过脱卤杂芳化途径而不是通过Hugerschoff途径生成2-氨基苯并噻唑涉及亲电取代反应。对于含有反应性邻卤素(例如–Br,–I)的硫脲,反应在室温下进行,通过脱卤途径生成2-氨基苯并噻唑,需要催化量的Cu(II)。没有转变硫脲用Cu(I)盐观察到(Tu)至Tag,表明该氧化转化需要氧化Cu(II)盐。温和的反应条件,对环境有益的试剂和溶剂,高收率,对各种官能团的耐受性是该方法的一些基本特征。DOI:10.1039/c2ra22240j
文献信息
-
Copper-Catalyzed Oxidative Amination of Benzoxazoles via C−H and C−N Bond Activation: A New Strategy for Using Tertiary Amines as Nitrogen Group Sources作者:Shengmei Guo、Bo Qian、Yinjun Xie、Chungu Xia、Hanmin HuangDOI:10.1021/ol1030298日期:2011.2.4method for oxidative amination of azoles with tertiary amines via copper-catalyzed C−H and C−N bond activation has been developed. This protocol can be performed in the absence of external base and only requires atmospheric oxygen as oxidant. The catalyst system is very simple and efficient, which opens a new way for using tertiary amines as nitrogen group sources for C−N bond formation reactions.
-
Pd-PEPPSI-IPent<sup>An</sup> Promoted Deactivated Amination of Aryl Chlorides with Amines under Aerobic Conditions作者:Fei-Dong Huang、Chang Xu、Dong-Dong Lu、Dong-Sheng Shen、Tian Li、Feng-Shou LiuDOI:10.1021/acs.joc.8b01205日期:2018.8.17We report herein a highly efficient Pd-catalyzed amination by “bulky-yet-flexible” Pd-PEPPSI-IPentAn complexes. The relationship between the N-heterocyclic carbenes (NHCs) structure and catalytic properties was discussed. Sterically hindered (hetero)aryl chlorides and a variety of aliphatic and aromatic amines can be applied in this cross-coupling, which smoothly proceeded to provide desired products
-
Well‐Defined Allylnickel Chloride/N‐Heterocyclic Carbene [(NHC)Ni(allyl)Cl] Complexes as Highly Active Precatalysts for CN and CS Cross‐Coupling Reactions作者:María José Iglesias、Auxiliadora Prieto、M. Carmen NicasioDOI:10.1002/adsc.201000223日期:2010.10.9The room temperature Buchwald–Hartwig amination of heteroaromatic chlorides has been achieved using the sterically bulky allylnickel chloride/N-heterocyclic carbene [(IPr)Ni(allyl)Cl] complex as a well-defined precatalyst. Arylation of aromatic thiols, affording high yields of products under low catalyst loadings, has also been promoted with the same complex.
-
Catalyst- and Reagent-Free Electrochemical Azole C−H Amination作者:Youai Qiu、Julia Struwe、Tjark H. Meyer、João C. A. Oliveira、Lutz AckermannDOI:10.1002/chem.201802832日期:2018.9.3Catalyst‐ and chemical oxidant‐free electrochemical azole C−H aminations were accomplished via cross‐dehydrogenative C−H/N−H functionalization. The catalyst‐free electrochemical C−H amination proved feasible on azoles with high levels of efficacy and selectivity, avoiding the use of stoichiometric oxidants under ambient conditions. Likewise, the C(sp3)−H nitrogenation proved viable under otherwise
-
[EN] PURINE DERIVATIVES AS KINASE INHIBITORS<br/>[FR] DERIVES DE LA PURINE EN TANT QU'INHIBITEURS DE LA KINASE申请人:LILLY CO ELI公开号:WO2003076442A1公开(公告)日:2003-09-18The present invention provides kinase inhibitors of Formula I.本发明提供了化合物I的激酶抑制剂。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
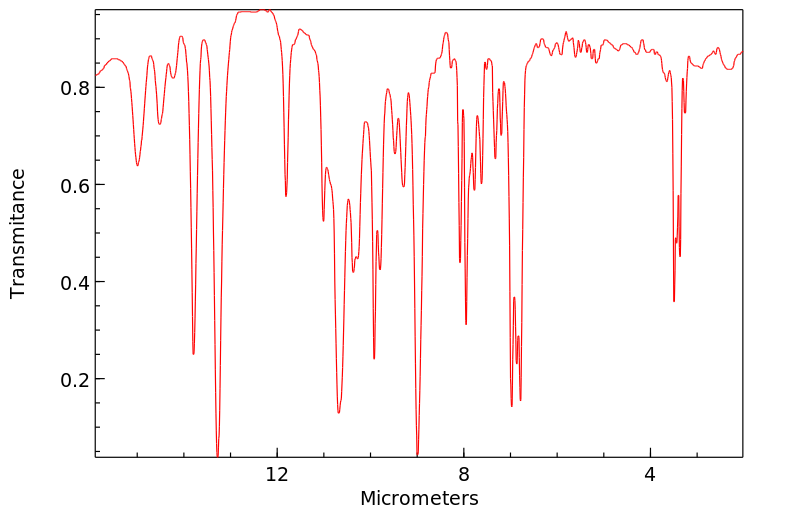
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1Z)-1-(3-乙基-5-羟基-2(3H)-苯并噻唑基)-2-丙酮
齐拉西酮砜
齐帕西酮-d8
阳离子蓝NBLH
阳离子荧光黄4GL
锂2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
铜酸盐(4-),[2-[2-[[2-[3-[[4-氯-6-[乙基[4-[[2-(硫代氧代)乙基]磺酰]苯基]氨基]-1,3,5-三嗪-2-基]氨基]-2-(羟基-kO)-5-硫代苯基]二氮烯基-kN2]苯基甲基]二氮烯基-kN1]-4-硫代苯酸根(6-)-kO]-,(1:4)氢,(SP-4-3)-
铜羟基氟化物
钾2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
钠3-(2-{(Z)-[3-(3-磺酸丙基)-1,3-苯并噻唑-2(3H)-亚基]甲基}[1]苯并噻吩并[2,3-d][1,3]噻唑-3-鎓-3-基)-1-丙烷磺酸酯
邻氯苯骈噻唑酮
西贝奈迪
螺[3H-1,3-苯并噻唑-2,1'-环戊烷]
螺[3H-1,3-苯并噻唑-2,1'-环己烷]
葡萄属英A
草酸;N-[1-[4-(2-苯基乙基)哌嗪-1-基]丙-2-基]-2-丙-2-基氧基-1,3-苯并噻唑-6-胺
苯酰胺,N-2-苯并噻唑基-4-(苯基甲氧基)-
苯酚,3-[[2-(三苯代甲基)-2H-四唑-5-基]甲基]-
苯胺,N-(3-苯基-2(3H)-苯并噻唑亚基)-
苯碳杂氧杂脒,N-1,2-苯并异噻唑-3-基-
苯甲酸,4-(6-辛基-2-苯并噻唑基)-
苯甲基2-甲基哌啶-1,2-二羧酸酯
苯并噻唑正离子,2-[3-(1,3-二氢-1,3,3-三甲基-2H-吲哚-2-亚基)-1-丙烯-1-基]-3-乙基-,碘化(1:1)
苯并噻唑正离子,2-[2-[4-(二甲氨基)苯基]乙烯基]-3-乙基-6-甲基-,碘化
苯并噻唑正离子,2-[(2-乙氧基-2-羰基乙基)硫代]-3-甲基-,溴化
苯并噻唑啉
苯并噻唑三氯金(III)
苯并噻唑-d4
苯并噻唑-7-乙酸
苯并噻唑-6-腈
苯并噻唑-5-羧酸
苯并噻唑-5-硼酸频哪醇酯
苯并噻唑-4-醛
苯并噻唑-4-乙酸
苯并噻唑-2-磺酸钠
苯并噻唑-2-磺酸
苯并噻唑-2-磺酰氟
苯并噻唑-2-甲醛
苯并噻唑-2-甲酸
苯并噻唑-2-甲基甲胺
苯并噻唑-2-基磺酰氯
苯并噻唑-2-基甲基-乙基-胺
苯并噻唑-2-基叠氮化物
苯并噻唑-2-基-邻甲苯-胺
苯并噻唑-2-基-己基-胺
苯并噻唑-2-基-(4-氯-苯基)-胺
苯并噻唑-2-基-(4-氟-苯基)-胺
苯并噻唑-2-基-(4-乙氧基-苯基)-胺
苯并噻唑-2-基-(2-甲氧基-苯基)-胺
苯并噻唑-2-基-(2,6-二甲基-苯基)-胺