3-甲氧基苯基乙酸 | 5451-83-2
中文名称
3-甲氧基苯基乙酸
中文别名
3-甲氧基乙酸苯酯
英文名称
acetic acid 3-methoxyphenyl ester
英文别名
3-Methoxyphenyl acetate;(3-methoxyphenyl) acetate
CAS
5451-83-2
化学式
C9H10O3
mdl
MFCD00026193
分子量
166.177
InChiKey
QQYNGKGFOZQMHD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1313.4
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
安全说明:S24/25
-
海关编码:2915390090
SDS
上下游信息
反应信息
-
作为反应物:描述:3-甲氧基苯基乙酸 在 aluminum (III) chloride 作用下, 反应 3.5h, 以75%的产率得到丹皮酚参考文献:名称:4-(1,2,3-三唑-1-基)香豆素衍生物作为潜在抗肿瘤药的合成及生物学评价摘要:在这项研究中,合成了一系列4-(1,2,3-三唑-1-基)香豆素结合物,并在体外评估了它们对三种人类癌细胞系(包括人类乳腺癌MCF-7细胞)的抗癌活性,结肠癌SW480细胞和肺癌A549细胞。为了提高生物学效能,进行了结构优化,重点是1,2,3-三唑的C-4位和香豆素的C-6,C-7位。此外,为进一步评估1,2,3-三唑和香豆素在抗增殖活性中的作用,还合成了9种具有4-(哌嗪-1-基)香豆素骨架的化合物和3种以喹啉核心为衍生物的化合物。通过体外MTT分析,大多数化合物显示出有吸引力的抗肿瘤活性,尤其是23。进一步的流式细胞术分析表明,化合物23通过阻止G 2 / M细胞周期并诱导凋亡而发挥抗增殖作用。DOI:10.1016/j.bmcl.2013.12.095
-
作为产物:描述:参考文献:名称:m-和p-取代苯乙酮的取代基效应 inino-Nitroperbenzoic Acid 氧化摘要:间位和对位取代苯乙酮(取代基:H、p-MeO、pt-Bu、pi-Pr、p-Et、p-Me、p-Cl、p-Br、m-MeO、 m-Me, m-Cl) 与邻硝基过苯甲酸在 30°C 的氯仿中进行研究。一般酸催化的速率常数是在几个浓度的邻硝基苯甲酸下测量的,邻硝基苯甲酸作为酸催化剂。获得的未催化和酸催化的速率常数分别为 σ 提供了 -2.16 和 -4.11 的 ρ 值。结果表明,对于所有研究的取代基而言,决定速率的步骤是过氧酸 - 羰基加合物中苯基的迁移,无论该反应是否为酸催化,并且酸催化剂仅干预形成酸-酮加合物处于初始状态而不是迁移步骤。DOI:10.1246/bcsj.64.2766
文献信息
-
Acetylation of alcohols and phenols by zinc zirconium phosphate as an efficient heterogeneous catalyst under solvent-free conditions作者:Abdol Reza Hajipour、Hirbod Karimi、Morteza KarimzadehDOI:10.1007/s00706-014-1222-9日期:2014.9AbstractAn efficient method for the acetylation of a wide range of alcohols as well as phenols with acetic anhydride in good to excellent yields under solvent-free conditions, using zinc zirconium phosphate as the catalyst was investigated. The catalyst was characterized by XRD, inductivity coupled plasma-optical emission spectroscopy, and scanning electron microscope. Products are easily isolated
-
Phosphomolybdic Acid: Mild and Efficient Catalyst for Acetylation of Alcohols, Phenols, and Amines under Solvent-Free Conditions作者:Sung Kim、Santosh KadamDOI:10.1055/s-2007-1000859日期:2008.1simple and efficient catalyst for the acetylation of alcohols, phenols, and amines. Acetylation reactions with acetic anhydride (1.0 equiv) proceed in excellent yield in the presence of a catalytic amount (0.2 mol%) of PMA at ambient temperature within a relatively short reaction time (<10 min). Structurally diverse alcohols, phenols, and amines undergo acetylation under solvent-free conditions.
-
Die Reduktion von β-Benzoyl-propionsäuren aus der Reihe des Resorcins作者:F. Zymalkowski、J. GelbergDOI:10.1002/ardp.19662990614日期:——mit NaBH4 bis zur Stufe der Hydroxysaure reduzieren. Die Ursachen werden diskutiert. Die elektrolytische Reduktion von Succinoylphenolen an einer Bleikathode liefert neben γ-Aryl-γ-hydroxybuttersauren deren pinacolartige Dimerisate, die durch Protonenkatalyse lactonisiert werden. 4-Methoxyphenyl-butyrolacton kann mit BBr3 entmethyliert werden. Die Reaktion last sich nicht auf 4-Methoxy-2-hydroxyphenyl-butyrolacton
-
Thallium(III) Chloride: A Mild and Efficient Catalyst for Acylation of Alcohols, Phenols and Thiols, and for Geminal Diacylation of Aldehydes under Solvent-Free Conditions作者:Sung Kim、Santosh KadamDOI:10.1055/s-0028-1083148日期:——simple and efficient catalyst for acylation of alcohols, phenols and thiols. It is also very effective for geminal diacylation of aldehydes. The acylation reaction using acetic anhydride proceeds in excellent yield in the presence of catalytic amounts ofthallium(III) chloride (1 mol%) at room temperature within relatively short reaction times (<20 min). Structurally diverse alcohols, phenols, thiols and
-
Benzothiazepines申请人:Roussel Uclaf公开号:US05063225A1公开(公告)日:1991-11-05A compound selected from the group consisting of all possible isomeric forms, racemic or optically active of a compound of the formula ##STR1## wherein the substituents are defined in the specification, and their non-toxic, pharmaceutically acceptable acid addition salts having antiarhythmic activity.从所有可能的同分异构体、消旋体或光学活性化合物中选择的一种化合物,其化学式为##STR1##,其中取代基在规范中定义,并且其无毒、药用可接受的酸盐具有抗心律失常活性。
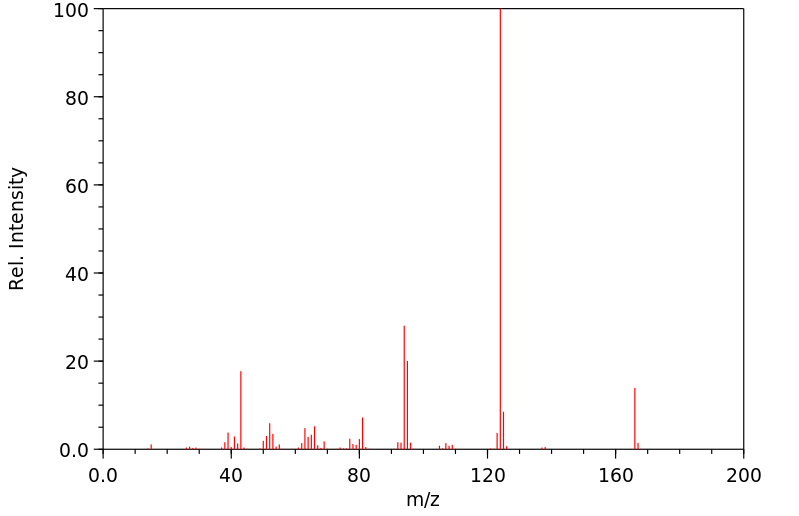
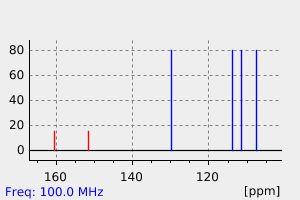
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰亚胺四聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺六聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺-酰胺-PEG8-四氟苯酚酯
马来酰亚胺-四聚乙二醇-五氟苯酯
马来酰亚胺-三聚乙二醇-五氟苯酚酯
靛酚乙酸酯
阿立哌唑标准品002
间硝基苯基戊酸酯
间氯苯乙酸乙酯
间乙酰苯甲酸
钾4-乙酰氧基苯磺酸酯
酚醛乙酸酯
邻苯二酚二乙酸酯
邻甲苯基环己甲酸酯
邻甲氧基苯乙酸酯
辛酸苯酯
辛酸对甲苯酚酯
辛酸五氯苯基酯
辛酸-(3-氯-苯基酯)
辛酰溴苯腈
苯酰胺,3,4-二(乙酰氧基)-N-[6-氨基-1,2,3,4-四氢-1-(4-甲氧苯基)-3-甲基-2,4-二羰基-5-嘧啶基]-
苯酚-乳酸
苯酚,4-异氰基-,乙酸酯(ester)
苯酚,4-[(四氢-2H-吡喃-2-基)氧代]-,乙酸酯
苯酚,3-(1,1-二甲基乙基)-,乙酸酯
苯酚,2-溴-3-(二溴甲基)-5-甲氧基-,乙酸酯
苯甲醇,4-(乙酰氧基)-3,5-二甲氧基-
苯甲酸,4-(乙酰氧基)-2-氟-
苯氧基氯乙酸苯酯
苯基金刚烷-1-羧酸酯
苯基氰基甲酸酯
苯基庚酸酯
苯基庚-6-炔酸酯
苯基己酸酯
苯基呋喃-2-羧酸酯
苯基吡啶-2-羧酸酯
苯基十一碳-10-烯酸酯
苯基乙醛酸酯
苯基乙酸酯-d5
苯基丙二酸单苯酯
苯基丙-2-炔酸酯
苯基丁-2,3-二烯酸酯
苯基4-乙基环己烷羧酸
苯基3-乙氧基-3-亚氨基丙酸盐
苯基2-(苯磺酰基)乙酸酯
苯基2-(4-甲氧基苯基)乙酸酯
苯基2-(2-甲氧基苯基)乙酸酯
苯基2-(2-甲基苯基)乙酸酯
苯基-乙酸-(2-甲酰基-苯基酯)
苯基-乙酸-(2-环己基-苯基酯)