(E)-N’-(4'-(dimethylamino)benzylidene)isonicotinohydrazide | 270576-24-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:20
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.13
-
拓扑面积:57.6
-
氢给体数:1
-
氢受体数:4
上下游信息
反应信息
-
作为反应物:参考文献:名称:Acylhydrazones as Widely Tunable Photoswitches摘要:Molecular photoswitches have attracted much attention in biological and materials contexts. Despite the fact that existing classes of these highly interesting functional molecules have been heavily investigated and optimized, distinct obstacles and inherent limitations remain. Considerable synthetic efforts and complex structure property relationships render the development and exploitation of new photoswitch families difficult. Here, we focus our attention on acylhydrazones: a novel, yet underexploited class of photochromic molecules based on the imine structural motif. We optimized the synthesis of these potent photoswitches and prepared a library of over 40 compounds, bearing different substituents in all four crucial positions of the backbone fragment, and conducted a systematic study of their photochromic properties as a function of structural variation. This modular family of organic photoswitches offers a unique combination of properties and the compounds are easily prepared on large scales within hours, through an atom-economic synthesis, from commercially available starting materials. During our thorough spectroscopic investigations, we identified photoswitches covering a wide range of thermal half-lives of their (Z)-isomers, from short-lived T-type to thermally stable P-type derivatives. By proper substitution, excellent band separation between the absorbance maxima of (E)- and (Z)-isomers in the UV or visible region could be achieved. Our library furthermore includes notable examples of rare negative photochromic systems, and we show that acylhydrazones are highly fatigue resistant and exhibit good quantum yields.DOI:10.1021/jacs.5b09519
-
作为产物:参考文献:名称:Acylhydrazones as Widely Tunable Photoswitches摘要:Molecular photoswitches have attracted much attention in biological and materials contexts. Despite the fact that existing classes of these highly interesting functional molecules have been heavily investigated and optimized, distinct obstacles and inherent limitations remain. Considerable synthetic efforts and complex structure property relationships render the development and exploitation of new photoswitch families difficult. Here, we focus our attention on acylhydrazones: a novel, yet underexploited class of photochromic molecules based on the imine structural motif. We optimized the synthesis of these potent photoswitches and prepared a library of over 40 compounds, bearing different substituents in all four crucial positions of the backbone fragment, and conducted a systematic study of their photochromic properties as a function of structural variation. This modular family of organic photoswitches offers a unique combination of properties and the compounds are easily prepared on large scales within hours, through an atom-economic synthesis, from commercially available starting materials. During our thorough spectroscopic investigations, we identified photoswitches covering a wide range of thermal half-lives of their (Z)-isomers, from short-lived T-type to thermally stable P-type derivatives. By proper substitution, excellent band separation between the absorbance maxima of (E)- and (Z)-isomers in the UV or visible region could be achieved. Our library furthermore includes notable examples of rare negative photochromic systems, and we show that acylhydrazones are highly fatigue resistant and exhibit good quantum yields.DOI:10.1021/jacs.5b09519
文献信息
-
Thiazolidin-4-one, azetidin-2-one and 1,3,4-oxadiazole derivatives of isonicotinic acid hydrazide: Synthesis and their biological evaluation作者:Sadaf Gilani、Suroor Khan、Ozair Alam、Vijender Singh、Alka AroraDOI:10.2298/jsc101104092g日期:——
A series of thiazolidin-4-one (2a-h, 3a-h), azetidin-2-one (4a- h) and 1,3,4-oxadiazole (5a-h) derivatives of isoninicotinic acid hydrazide (INH) were synthesized in order to obtain new compounds with potential anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities. The structures of the new compounds were supported by their IR, 1H-NMR and mass spectral data. All compounds were evaluated for their anti-inflammatory activity by the carrageenan-induced rat paw edema test method. Eleven of the new compounds, out of 32, showed very good anti-inflammatory activity in the carrageenan-induced rat paw edema test, with significant analgesic activity in the tail immersion method together with negligible ulcerogenic action. The compounds, which showed less ulcerogenic action, also showed reduced malondialdehyde content (MDA), which is one of the by-products of lipid peroxidation. The study showed that the compounds inhibited the induction of gastric mucosal lesions and it can be suggested from the results that their protective effects may be related to inhibition of lipid peroxidation in the gastric mucosa.
一系列噻唑烷-4-酮(2a-h,3a-h)、氮杂环丁烷-2-酮(4a-h)和 异烟酸酰肼(INH)的一系列噻唑烷-4-酮(2a-h,3a-h)、氮杂环丁烷-2-酮(4a-h)和 1,3,4-噁二唑(5a-h)衍生物的 合成了异烟酸酰肼(INH)衍生物,以获得具有潜在 新化合物具有潜在的抗炎、镇痛、致溃疡和脂质过氧化活性。 新化合物的结构得到了红外光谱、1H-NMR 和 质谱数据支持了新化合物的结构。所有化合物的抗炎活性都通过角叉菜胶诱导的 抗炎活性。在 32 个新化合物中 在卡拉胶诱导的大鼠爪水肿试验中表现出很好的抗炎活性。 在角叉菜胶诱导的大鼠爪水肿试验中表现出很好的抗炎活性,在尾部浸泡法中表现出显著的镇痛活性,同时还表现出很强的抗菌活性。 在尾部浸泡法中具有显著的镇痛活性,而致溃疡作用则微乎其微。 致溃疡作用较弱的化合物还显示出较低的 丙二醛含量(MDA)。 过氧化物。研究表明,这些化合物抑制了诱导的 研究结果表明,这些化合物抑制了胃黏膜病变的诱导。 保护作用可能与抑制胃黏膜脂质过氧化有关。 胃黏膜的脂质过氧化作用有关。 -
Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole##This paper represents contribution # 36 of the LASSBio, UFRJ (Br.) (LASSBio, http://acd.ufrj.br/≈pharma/lassbio); For contribution # 35, see [24].作者:Patrícia C. Lima、Lídia M. Lima、Kelli Cristine M. da Silva、Paulo Henrique O. Léda、Ana Luisa P. de Miranda、Carlos A.M. Fraga、Eliezer J. BarreiroDOI:10.1016/s0223-5234(00)00120-3日期:2000.2Anew series of antinociceptive compounds belonging to the N-acylarylhydrazone (NAH) class were synthesized from natural safrole (7). The most analgesic derivative represented by 10f, [(4'-N,N-dimethylaminobenzylidene-3-(3', 4'-methylenedioxyphenyl)propionylhydrazine], was more potent than dipyrone and indomethacin, used as standards. The NAH compounds described herein were structurally planned by molecular
-
Synthesis, Characterization of (E)-N'-(substituted-benzylidene)isonicotinohydrazide Derivatives as Potent Antitubercular Agents作者:Manav Malhotra、Rajiv Sharma、Vikramdeep Monga、Aakash Deep、Kapendra Sahu、Abdul SamadDOI:10.2174/157018011795906866日期:2011.7.1A series of 19 isonicotinic acid hydrazide derivatives has been synthesized and evaluated for their in vitro antitubercular activity against Mycobacterium tuberculosis H37Rv using alamar blue susceptibility test. The synthesized compounds inhibit Mycobacterium tuberculosis H37Rv strain with minimum inhibitory concentration ranging from (0.00014-0.01174 mM). Among all synthesized compounds seven derivatives 2a, 2b, 2e, 2h, 2l, 2m and 2q were more potent than isoniazid and the compound 2q emerged as the most potent derivative, being more effective than isoniazid with an (MIC 0.00023 mM) in vitro. The results demonstrated the potential and importance of developing new isoniazid derivatives against Mycobacterium infections.
-
Anti-infective compounds申请人:Brodin Priscille公开号:US20110178077A1公开(公告)日:2011-07-21The present invention relates to small molecule compounds and their use in the treatment of bacterial infections, in particular Tuberculosis.本发明涉及小分子化合物及其在治疗细菌感染,特别是结核病方面的用途。
-
Xanthine oxidase inhibitory activity of nicotino/isonicotinohydrazides: A systematic approach from in vitro , in silico to in vivo studies作者:Humaira Zafar、Muhammad Hayat、Sumayya Saied、Momin Khan、Uzma Salar、Rizwana Malik、M. Iqbal Choudhary、Khalid Mohammed KhanDOI:10.1016/j.bmc.2017.02.044日期:2017.4involved in regulation of serum uric acid, xanthine oxidase (XO) is the best pharmacological target to control the levels of serum uric acid as it catalyzes the final steps in uric acid production. In the current study, a systemic search for the inhibitors of xanthine oxidase, starting from synthesis to in vitro screening and leading to in vivo studies is presented. Benzylidene nicotino/isonicotinohydrazides生活方式和饮食习惯的改变导致全球高尿酸血症的患病率增加。高尿酸血症的作用不再局限于痛风,而在CVD,高血压,代谢综合征和关节炎的进展中起着核心作用。在涉及调节血清尿酸的不同因素中,黄嘌呤氧化酶(XO)是控制血清尿酸水平的最佳药理学靶标,因为它催化了尿酸生产的最终步骤。在当前的研究中,从合成到体外筛选,再到体内研究,对黄嘌呤氧化酶抑制剂进行了系统的研究。通过用取代的芳族醛处理烟碱/异烟碱酰肼来合成苄叉烟碱/异烟碱酰肼(1-54),并通过EI-MS和1H NMR进行表征。还进行了元素分析。首先使用体外光谱XO抑制试验筛选所有合成化合物的黄嘌呤氧化酶抑制活性。与标准药物别嘌呤醇IC50 = 2.00±0.01μM相比,发现其中22种衍生物的IC50值为0.96至330.4μM。对五种活性最高的化合物(8、35、36、39和45)进行了动力学研究,其IC50值在0.96至54.8μM之间,具有抑制
表征谱图
-
氢谱1HNMR
-
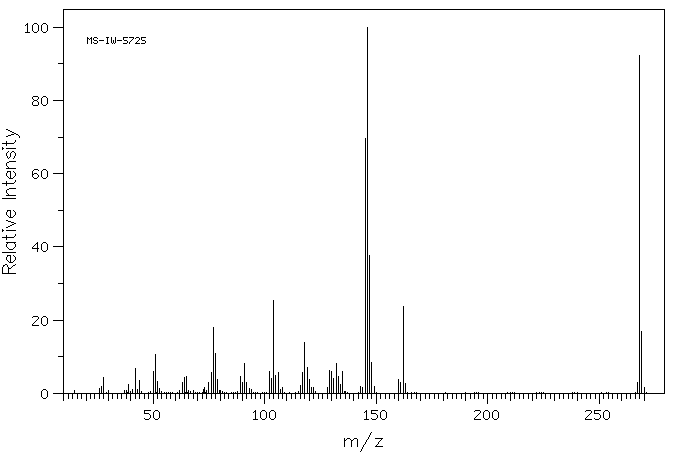
质谱MS
-
碳谱13CNMR
-
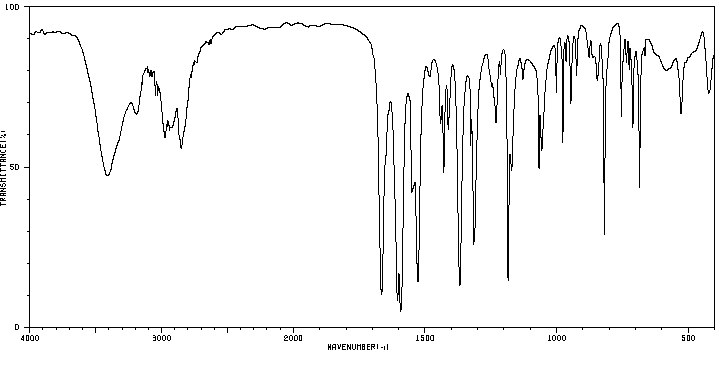
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息