1,2-二异烟酰基肼 | 4329-75-3
中文名称
1,2-二异烟酰基肼
中文别名
——
英文名称
N,N'-bis(isonicotinoyl)hydrazine
英文别名
N'-isonicotinoylisonicotinohydrazide;N,N'-bis(4-picolinoyl)hydrazine;1,2-diisonicotinoylhydrazine;1,2-isonicotinoylhydrazine;Iso-INH;N,N'-diisonicotinoyl-hydrazine;N'-(pyridine-4-carbonyl)pyridine-4-carbohydrazide
CAS
4329-75-3
化学式
C12H10N4O2
mdl
MFCD00534270
分子量
242.237
InChiKey
HDADCMZPLLONGB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:250 °C
-
沸点:551.5±38.0 °C(Predicted)
-
密度:1.313±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(轻微)、甲醇(轻微、加热)
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:18
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:84
-
氢给体数:2
-
氢受体数:4
安全信息
-
海关编码:2933399090
-
包装等级:III
-
危险类别:4.1
-
危险性防范说明:P240,P210,P241,P280,P370+P378
-
危险品运输编号:1325
-
危险性描述:H228
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异烟肼 isoniazid 54-85-3 C6H7N3O 137.141
反应信息
-
作为反应物:描述:参考文献:名称:使用劳森试剂合成取代的 1,3,4-噻二唑摘要:含硫化合物具有许多特殊和独特的性质,这使它们继续受到关注。由于其广泛的生物活性谱,1,3,4-噻二唑已被检查为潜在的抗菌剂,“抗病毒?镇痛剂:抗肿瘤剂:抗惊厥药、消炎药~、药物、杀虫剂和杀菌剂。其中一些形成热致液晶并具有有趣的电光特性。6 此外,噻二唑已被用作腐蚀和氧化抑制剂^,^染料或金属离子络合剂~。~ ~ J ~ J 这类化合物最常用的合成方法包括硫酰肼或其他底物与 SCNNCS 部分的环化和脱水。'2,6 通常在此类反应中使用磷酰氯或硫酸。1,3,4-噻二唑环合成的另一种途径是通过将 1,3,4-恶二唑中的氧原子交换为硫,使用十硫化四磷或硫脲;I2 但是根据我们的经验,这种方法在许多情况下不起作用案件。Lawesson 描述了 1 ,2-二酰基肼与 2,4 双(4-甲氧基苯基)1,2,3,4-二噻二膦(劳森试剂)的硫化作用,然后是自发环化和脱氢硫化,这是一种改进的噻二唑环形成方法。DOI:10.1080/00304940509354950
-
作为产物:描述:异烟肼 在 copper diacetate 作用下, 反应 0.07h, 以85%的产率得到1,2-二异烟酰基肼参考文献:名称:在无溶剂条件下在微波辐射下使用乙酸铜 (II) 将酸酰肼高效氧化成 N,N'-二酰基肼摘要:摘要 描述了在微波辐射下使用乙酸铜 (II) 将酸酰肼 1 的无溶剂“干”态转化为相应的 N,N '-二酰基肼 2 的一种简单有效的方法。以良好的收率和优良的纯度获得产品。DOI:10.1081/scc-120025183
文献信息
-
Synthesis, computational studies, antimycobacterial and antibacterial properties of pyrazinoic acid–isoniazid hybrid conjugates作者:Siva S. Panda、Adel S. Girgis、Bibhuti B. Mishra、Mohamed Elagawany、Venkatasai Devarapalli、William F. Littlefield、Ahmed Samir、Walid Fayad、Nehmedo G. Fawzy、Aladdin M. Srour、Riham M. BokhtiaDOI:10.1039/c9ra03380g日期:——Benzotriazole and microwave mediated syntheses led to a new set of hybrid conjugates of pyrazinoic acid with isoniazid via amino acid linkers in excellent yields with retention of chirality. Microbiological screening of the synthesized conjugates revealed an exceptionally high activity against some of the pathogenic bacterial strains at low concentrations. Promising antimycobacterial properties were
-
An Environmentally Benign Electrochemical Process for the Reduction of Carboxylic Acid Hydrazides to Amides作者:Rolf Breinbauer、Matthias Mentel、Matthias BeierDOI:10.1055/s-0028-1088164日期:——The transformation of acid hydrazides to primary amides is of certain relevance for the organic synthesis of complex molecules. While existing methods require harsh reaction conditions, we present an electrochemical approach in which monoacylhydrazines are reduced to primary amides in 40-90% yield in a divided electrochemical cell with a tin cathode. This method proved superior to reduction by sodium/mercury
-
Hypervalent Iodine Oxidation of Acid Hydrazides: A New Synthesis of<i>N,N</i>′-Diacylhydrazines作者:Om Prakash、Vijay Sharma、Anil SadanaDOI:10.1080/00397919708005637日期:1997.10Abstract Hypervalent iodine oxidation of p-substituted benzohydrazides (1a-h), phenylacetohydrazide (1i) and heterocyclic acid hydrazides (1j-I) using one equivalent of iodobenzene diacetate in dry dichloromethane or acetonitrile leads to dimerization thereby providing a new and facile method for the synthesis of N,N-diacylhydrazines 2.
-
Migration of an acyl group in the pyrazole system: synthesis of 1-acyl-3-hydroxy-1H-pyrazoles and related derivatives. A new preparation of N,N ′-diacylhydrazines作者:Vladimir Kepe、Franc Požgan、Amalija Golobič、Slovenko Polanc、Marijan KočevarDOI:10.1039/a803973i日期:——from 4-ethoxymethylene-2-phenyloxazol-5(4H)-one (1) and hydrazides, 4-phenylsemicarbazide, 4-phenylthiosemicarbazide and benzyl carbazate in boiling dioxane is described. The method includes a migration of an acyl or related unit. The X-ray study and NMR spectroscopic examination confirmed the structure of the products. A one-pot synthesis of N,N′-diacylhydrazines from hydrazides by the assistance of
-
The formation and double decomposition of pyridoxal isonicotinoylhydrazone dimethiodide mediated by iron(II) salts作者:Shalom Sarel、Schely Avramovici-Grisaru、Shmuel CohenDOI:10.1039/c39860000047日期:——Iron(II) ions are shown to induce a hitherto unknown nitrogen-centred metathesis (‘hydrazine metathesis’) of pyridoxal isonicotinoylhydrazone dimethiodide (1) into the symmetrical hydrazine derivatives pyridoxal azine dimethiodide (7) and dimethiodide of bis-isonicotinoyl hydrazide (8) together with isonicotinoylamide methiodide (9) in a ratio 2 : 1 : 1, respectively.
表征谱图
-
氢谱1HNMR
-
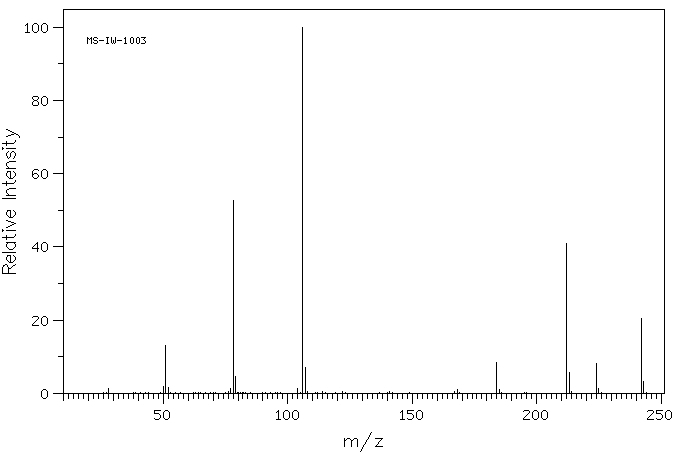
质谱MS
-
碳谱13CNMR
-
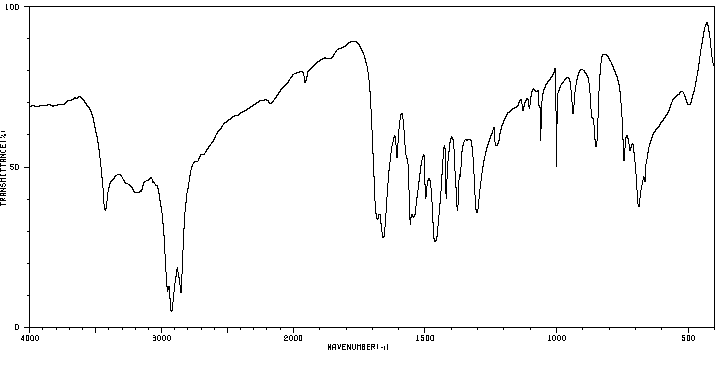
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-