1-甲基吲唑 | 13436-48-1
中文名称
1-甲基吲唑
中文别名
——
英文名称
1-methylindazole
英文别名
1-methyl-1H-indazole;N-methylindazole
CAS
13436-48-1
化学式
C8H8N2
mdl
MFCD00661658
分子量
132.165
InChiKey
CSUGQXMRKOKBFI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:60.5°C
-
沸点:231.05°C
-
密度:1.0315
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:17.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2933990090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1-Methyl-1H-indazole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-Methyl-1H-indazole
CAS number: 13436-48-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8N2
Molecular weight: 132.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1-Methyl-1H-indazole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-Methyl-1H-indazole
CAS number: 13436-48-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8N2
Molecular weight: 132.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-碘-1-甲基-1H-吲唑 3-iodo-1-methyl-1H-indazole 52088-10-5 C8H7IN2 258.061 1-甲基-1H-吲唑-3-胺 1-methyl-1H-indazol-3-amine 60301-20-4 C8H9N3 147.18 吲唑-1-乙酸 2-(1H-indazol-1-yl)acetic acid 32829-25-7 C9H8N2O2 176.175 3-溴-1-甲基吲唑 3-bromo-1-methyl-1H-indazole 326474-67-3 C8H7BrN2 211.061 吲唑 benzimidazole 271-44-3 C7H6N2 118.138 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-溴-1-甲基-1H-吲唑 5-bromo-1-methyl-1H-indazole 465529-57-1 C8H7BrN2 211.061 —— 5-chloro-1-methyl-1H-indazole 1379091-92-5 C8H7ClN2 166.61 1,3-二甲基-1H-吲唑 1,3-dimethyl-1H-indazole 34879-84-0 C9H10N2 146.192 1-甲基-1H-吲唑-3-胺 1-methyl-1H-indazol-3-amine 60301-20-4 C8H9N3 147.18 3-溴-1-甲基吲唑 3-bromo-1-methyl-1H-indazole 326474-67-3 C8H7BrN2 211.061 3-氯-1-甲基-1H-吲唑 3-chloro-1-methyl-1H-indazole 52354-75-3 C8H7ClN2 166.61 —— 1-[bis(trimethylsilyl)methyl]indazole 300808-13-3 C14H24N2Si2 276.529 1-甲基-1H-吲唑-3-甲腈 1-methyl-1H-indazole-3-carbonitrile 31748-44-4 C9H7N3 157.175 3,5-二溴-1-甲基-1H-吲唑 3,5-dibromo-1-methyl-1H-indazole 52088-11-6 C8H6Br2N2 289.957 —— 3,5-dichloro-1-methyl-1H-indazole 1446407-29-9 C8H6Cl2N2 201.055
反应信息
-
作为反应物:描述:1-甲基吲唑 在 potassium phosphate 、 2,6-二叔丁基吡啶 、 (1,5-cyclooctadiene)(methoxy)iridium(I) dimer 、 甲烷磺酸(2-二环己基膦基-2',4',6'-三-异丙基-1,1'-联苯基)(2'-氨基-1,1'-联苯-2-基)钯(II) 作用下, 以 甲基叔丁基醚 、 水 为溶剂, 反应 1.83h, 生成 4-(1-methyl-1H-indazol-3-yl)phenol参考文献:名称:Synthesis of 3-aryl-1H-indazoles via iridium-catalysed C–H borylation and Suzuki–Miyaura coupling摘要:实现了1H-吲唑的区域选择性铱催化C3-硼化反应。随后,硼酸酯与芳基氯、溴和碘的Suzuki-Miyaura偶联反应以良好的产率得到3-芳基-1H-吲唑。DOI:10.1039/c4ra04235b
-
作为产物:描述:参考文献:名称:v. Auwers; Dueesberg, Chemische Berichte, 1920, vol. 53, p. 1204摘要:DOI:
-
作为试剂:描述:4-(7-(3,4-dimethoxyphenylamino)thiazolo[5,4-d]pyrimidin-5-yl)benzoic acid 、 N,N-二甲基乙二胺 在 1-甲基吲唑 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 盐酸 作用下, 以 二氯甲烷 、 甲醇 为溶剂, 反应 3.25h, 以25%的产率得到4-(7-(3,4-dimethoxyphenylamino)thiazolo[5,4-d]pyrimidin-5-yl)-N-(2-(dimethylamino)ethyl)benzamide hydrochloride参考文献:名称:THIAZOLOPYRIMIDINE COMPOUNDS摘要:本发明涉及使用以下公式I的新型吡咯并吡嗪衍生物: 其中所有可变取代基均如本文所述定义,这些衍生物是SYK抑制剂,可用于治疗自身免疫性和炎症性疾病。公开号:US20120252777A1
文献信息
-
[EN] HETEROCYCLIC COMPOUNDS FOR THE TREATMENT OF STRESS-RELATED CONDITIONS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES POUR LE TRAITEMENT D'ÉTATS LIÉS AU STRESS申请人:OTSUKA PHARMA CO LTD公开号:WO2010137738A1公开(公告)日:2010-12-02The present invention provides a novel heterocyclic compound. A heterocyclic compound represented by general formula (1) wherein, R1 and R2, each independently represent hydrogen; a phenyl lower alkyl group that may have a substituent(s) selected from the group consisting of a lower alkyl group and the like on a benzene ring and/or a lower alkyl group; or a cyclo C3-C8 alkyl lower alkyl group; or the like; R3 represents a lower alkynyl group or the like; R4 represents a phenyl group that may have a substituent(s) selected from the group consisting of a 1,3,4-oxadiazolyl group that may have e.g., halogen or a heterocyclic group selected from pyridyl group and the like; the heterocyclic group may have at least one substituent(s) selected from a lower alkoxy group and the like or a salt thereof.
-
[EN] TRIAZOLOPYRIDINE INHIBITORS OF MYELOPEROXIDASE<br/>[FR] INHIBITEURS, À BASE DE TRIAZOLOPYRIDINE, DE LA MYÉLOPEROXYDASE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2017040451A1公开(公告)日:2017-03-09The present invention provides compounds of Formula (I): wherein A is as defined in the specification, and compositions comprising any of such novel compounds. These compounds are myeloperoxidase (MPO) inhibitors and/or eosinophil peroxidase (EPX) inhibitors, which may be used as medicaments.
-
Ligand-accelerated non-directed C–H functionalization of arenes作者:Peng Wang、Pritha Verma、Guoqin Xia、Jun Shi、Jennifer X. Qiao、Shiwei Tao、Peter T. W. Cheng、Michael A. Poss、Marcus E. Farmer、Kap-Sun Yeung、Jin-Quan YuDOI:10.1038/nature24632日期:2017.11challenges associated with the lack of sufficiently active palladium catalysts. Currently used palladium catalysts are reactive only with electron-rich arenes, unless an excess of arene is used, which limits synthetic applications. Here we report a 2-pyridone ligand that binds to palladium and accelerates non-directed C–H functionalization with arene as the limiting reagent. This protocol is compatible with碳氢键 (C-H) 的定向活化在合成有用反应的发展中很重要,因为通过配位官能团实现了邻近诱导的反应性和选择性。钯催化的非定向 C-H 活化有可能实现进一步有用的反应,因为它可以到达更远的位置并应用于不含适当定向基团的底物;然而,由于缺乏足够活性的钯催化剂,它的发展面临着巨大的挑战。目前使用的钯催化剂仅与富电子芳烃反应,除非使用过量的芳烃,这限制了合成应用。在这里,我们报告了一种 2-吡啶酮配体,它与钯结合并以芳烃为限制剂加速非定向 C-H 功能化。该协议与广泛的芳香底物兼容,我们展示了不能过量使用的高级合成中间体、药物分子和天然产物的直接功能化。我们还开发了 C-H 烯化和羧化方案,证明了我们的方法对其他转化的适用性。这些转化中的位点选择性受空间和电子效应的组合控制,吡啶酮配体增强了空间对选择性的影响,从而为定向 C-H 功能化提供了互补的选择性。该协议与广泛的芳香底物兼容,我们展示了不能
-
SULFONE AMIDE LINKED BENZOTHIAZOLE INHIBITORS OF ENDOTHELIAL LIPASE申请人:BRISTOL-MYERS SQUIBB COMPANY公开号:US20160326125A1公开(公告)日:2016-11-10The present invention provides compounds of Formula (I) as defined in the specification and compositions comprising any of such novel compounds. These compounds are endothelial lipase inhibitors which may be used medicaments.本发明提供了规范中定义的Formula (I)的化合物以及包含任何此类新化合物的组合物。这些化合物是内皮酶抑制剂,可用作药物。
-
[EN] MONOACYLGLYCEROL LIPASE MODULATORS<br/>[FR] MODULATEURS DE LA MONOACYLGLYCÉROL LIPASE申请人:JANSSEN PHARMACEUTICA NV公开号:WO2021191359A1公开(公告)日:2021-09-30Fused and bridged compounds of Formula (I), and pharmaceutically acceptable salts, isotopes, N-oxides, solvates, and stereoisomers thereof, pharmaceutical compositions containing them, methods of making them, and methods of using them including methods for treating disease states, disorders, and conditions associated with MGL modulation, such as those associated with pain, psychiatric disorders, neurological disorders (including, but not limited to major depressive disorder, treatment resistant depression, anxious depression, autism spectrum disorders, Asperger syndrome, bipolar disorder), cancers and eye conditions: (I) wherein R1a, R1b, R2, and R3, are defined herein.化合物的融合和桥联化合物的化学式(I),以及其药用可接受的盐、同位素、N-氧化物、溶剂合物和立体异构体,含有它们的药物组合物,制备它们的方法,以及使用它们的方法,包括用于治疗与MGL调节相关的疾病状态、紊乱和症状的方法,例如与疼痛、精神紊乱、神经紊乱(包括但不限于重度抑郁症、治疗抵抗性抑郁症、焦虑性抑郁症、自闭症谱系障碍、亚斯伯格综合症、双相情感障碍)、癌症和眼部疾病相关的方法:(I)其中R1a、R1b、R2和R3在此定义。
表征谱图
-
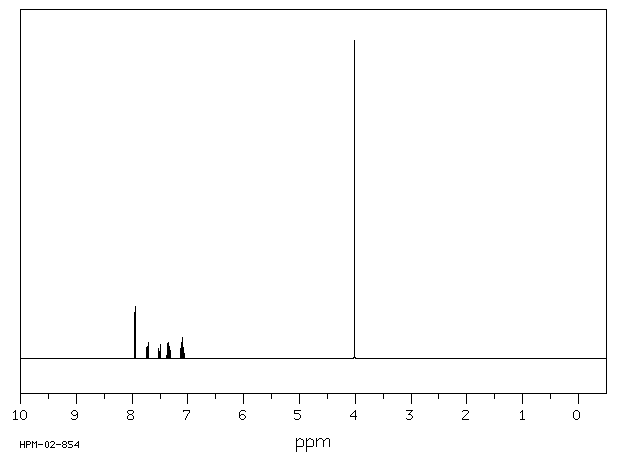
氢谱1HNMR
-
质谱MS
-
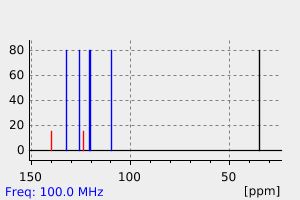
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙基N-(1H-吲唑-3-基羰基)ethanehydrazonoate)
(2R,6S)-2,6-二甲基-4-[6-[5-(1-(甲基环丙基)氧基]-1H-吲唑-3-基]嘧啶-4-基]吗啉
顺式-八氢-2-甲基-吡咯并[3,4-c]吡咯
陀尼达安
阿西替尼杂质
阿布查米卡代谢物M4
达泽达明
苯达扎克钠
苯甲酸,5-(溴甲基)-2-硝基-,甲基酯
苯乙胺杂质8
苯丙酰胺,b-氨基-3-乙氧基-
苄达酸钠盐
苄达酸
苄达明 N-氧化物
苄达明
苄胺N-氧化物马来酸氢盐
苄基-(3-氯-5-硝基-1H-吲唑-7-基)-胺
盐酸苄达明
1-(2,4-二氯苄基)-1H-吲唑-3-碳酰肼
甲基7-氨基-1H-吲唑-3-羧酸酯
甲基5-溴-1-(四氢-2H-吡喃-2-基)-1H-吲唑-3-甲酸基酯
甲基4-碘-1H-吲唑-6-羧酸
甲基4-氨基-1-乙基-1H-吲唑-6-羧酸酯
甲基-6-硝基-1H-吲唑-3-羧酸
甲基 1H-吲唑-7-羧酸盐酸盐
水杨酸化合物与3-[(1-苄基-1H-吲唑-3-基)氧基]-N,N-二甲基丙基胺(1:1)
氯尼达明
格拉斯琼杂质C
格拉司琼相关物质C
格拉司琼相关物质A
格拉司琼氮氧化物
帕唑帕尼相关化合物2
帕唑帕尼杂质57
希尼达明
宾达利
奈米利塞
奈拉米诺
因旦尼定
四溴甲基-1-甲基吲唑
哌啶,2-乙基-1-(3-苯基-2-丙炔-1-基)-
吲熟酯
吲唑-6-硼酸
吲唑-5-甲酸盐酸盐
吲唑-5-甲酸甲酯
吲唑-5-甲腈
吲唑-4-硼酸盐酸盐
吲唑-4-乙酸
吲唑-3-羧酸乙脂
吲唑-3-羧酸
吲唑-3-乙酸