N‑(2,4,6‑trinitrophenyl)naphthalen‑2‑amine | 33963-99-4
中文名称
——
中文别名
——
英文名称
N‑(2,4,6‑trinitrophenyl)naphthalen‑2‑amine
英文别名
N-2,4,6-Trinitrobenzo-2-naphthylamin;[2]naphthyl-picryl-amine;(2.4.6-Trinitro-phenyl)-β-naphthylamin;Pikryl-β-naphthylamin;N-(2,4,6-Trinitrophenyl)-2-naphthylamine;N-(2,4,6-trinitrophenyl)naphthalen-2-amine
CAS
33963-99-4
化学式
C16H10N4O6
mdl
——
分子量
354.279
InChiKey
RJBBULPFDKEBBZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:1680 °C
-
沸点:507.0±50.0 °C(Predicted)
-
密度:1.573±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.8
-
重原子数:26
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:150
-
氢给体数:1
-
氢受体数:7
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-甲基-2,4,6-三硝基苯胺 N-methylpicramide 1022-07-7 C7H6N4O6 242.148
反应信息
-
作为反应物:描述:N‑(2,4,6‑trinitrophenyl)naphthalen‑2‑amine 在 硫酸 、 tin(ll) chloride 作用下, 生成 benzo[a]phenazine-8,10-diyldiamine参考文献:名称:Kehrmann; Riera y Punti, Chemische Berichte, 1911, vol. 44, p. 2623摘要:DOI:
-
作为产物:描述:参考文献:名称:2,4,6-三硝基苯胺衍生物作为强效抗肿瘤药的合成及生物学评价摘要:摘要 含硝基基团的化合物是众所周知的有效抗癌药。该研究的目的是合成一系列三硝基苯胺衍生物,以确定其在多种癌细胞模型上的潜在抗肿瘤活性,在肝癌细胞上的抗凋亡和抗转移特性。抗增殖的研究表明,IC 50的值ñ -苯基-2,4,6- trinitroaniline,ñ - (2,4,6-三硝基苯基)萘-1-胺,ñ - (2,4,6-三硝基苯萘-2-胺,N-(3-硝基苯基)-2,4,6-三硝基苯胺与Hep3B细胞中顺铂的IC 50值相似。实际上,IC 50的N值-(3,5-二氟苯基)-2,4,6-三硝基苯胺比顺铂更好。此外,所有化合物均可降低细胞周期检查点蛋白cyclin D1的表达。为了研究化合物对细胞凋亡途径的影响,采用qRT-PCR和Western blot分析了Bcl-2和Bax的mRNA和蛋白表达。膜联蛋白V染色测定法,细胞凋亡的mRNA和蛋白质的分析表明,ñ -异丙基-2,4,6- trinitroaniline,ñDOI:10.1007/s00706-020-02690-7
文献信息
-
Okon, Roczniki Chemii, 1953, vol. 32, p. 213,218, 221, 222作者:OkonDOI:——日期:——
-
Seats That May Not Matter: Testing for Racial Polarization in U.S. City Councils作者:Rory Allan AustinDOI:10.3162/036298002x200693日期:2002.8.1
-
Okon; Kaminski, Biuletyn Wojskowej Akademii Technicznej imienia Jaroslawa Dabrowskiego, 1958, vol. 7, # 38, p. 35,42作者:Okon、KaminskiDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
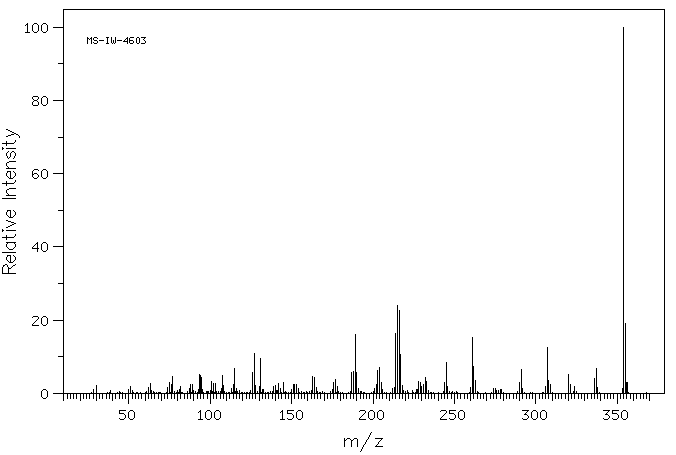
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮