二环[3.2.0]庚-6-烯 | 4927-03-1
中文名称
二环[3.2.0]庚-6-烯
中文别名
——
英文名称
bicyclo[3.2.0]hept-6-ene
英文别名
Δ6-bicyclo<3.2.0>heptane;bicyclo<3.2.0>hept-6-ene;Bicyclo<3.2.0>hept-6-en;{3,2,0}bicyclohept-6-ene;<13C>Bicyclo<3.2.0>hept-6-en;Δ6-Bicyclo<3.2.0>heptan
CAS
4927-03-1
化学式
C7H10
mdl
——
分子量
94.1564
InChiKey
LMULYBMQCQUKHQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:96-98 °C
-
密度:0.955±0.06 g/cm3(Predicted)
-
保留指数:1014
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:7
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— bicyclo[4.1.0]hept-2-ene 2566-57-6 C7H10 94.1564
反应信息
-
作为反应物:描述:二环[3.2.0]庚-6-烯 生成 (1S,2R,6R,7R)-4-[[(1S,2R,4R)-7,7-dimethyl-1-(methylsulfanylmethyl)-2-bicyclo[2.2.1]heptanyl]oxy]tricyclo[5.3.0.02,6]dec-4-en-3-one参考文献:名称:Verdaguer Xavier, Movano Albert, Pericas Miquel A., Riera Antoni, Bernard+, J. Amer. Chem. Soc, 116 (1994) N 5, S 2153-2154摘要:DOI:
-
作为产物:参考文献:名称:Kirmse,W.; Pook,K.-H., Chemische Berichte, 1965, vol. 98, p. 4022 - 4026摘要:DOI:
文献信息
-
syn-anti Isomerism in the 1,3-dipolar cycloaddition to cis-3,4-disubstituted cyclobutenes. Part 2. Role of conformation in bicyclo[3.2.0]hept-6-enes作者:Marina Burdisso、Remo Gandolfi、Paolo Pevarello、Augusto RastelliDOI:10.1039/p29880000753日期:——which, however, becomes flatter and flatter on passing from (1a) to (1e). As a result there is a progessive lessening of steric hindrance on the syn face which neatly parallels the observed increase in syn attack along the series (1a–e). Moreover the energy required to remove steric hindrance on passing from the boat-like to the half-planar conformation is definitively lower for (1c–e) than for (1b and双环[3.2.0]庚-6-烯(1a)和2,4-二恶双环[3.2.0]庚-6-烯(1b)与重氮甲烷和苯乙氧基环氧乙烷反应,仅得到反加合物。相反,从相同的1,3-偶极与双环[3.2.0] hept-6-en-3-one(1c),2,4-二氧杂双环[3.2 ]的反应中分离出了顺式和反式加合物的混合物0.0]庚-6-烯-3-酮(1E),甚至与显然最拥挤,在顺面,3,3-二甲基-2,4-二氧杂双环[3.2.0]庚-6-烯(1d)。广泛的从头算起MO计算(4-31G)表明化合物(1)更喜欢船形构象,但是从(1a)到(1e)时会变得越来越扁平。其结果上有空间位阻的progessive减轻顺面整齐地平行于所观察到的增加顺沿系列(攻击1A - ë)。此外,消除(1c – e)时从船形到半平面构象消除空间位阻所需的能量肯定比(1b和a)低。
-
cis,trans-Cyclohepta-1,3-diene as a transient intermediate in the photocyclization of cis,cis-cyclohepta-1,3-diene作者:Yoshihisa Inoue、Shoji Hagiwara、Yoshihiko Daino、Tadao HakushiDOI:10.1039/c39850001307日期:——The photocyclization of cis,cis-Cyclohepta-1,3-diene (1) to give bicyclo[3.2.0]hept-6-ene (2) proceed through a two-step process, in which the cis,cis-isomer (1) isomerizes photochemically to the highly strained cis,trans-cycloheptadiene (5) which in turn cyclizes thermally to give the bicycloheptene (2); the cis,trans-isomer (5) was trapped chemically by acidic methanol at room temperature to give
-
Studies on the Pauson–Khand reaction of alkynyl sulfoxides. Unexpectedly easy racemization of their dicobalt hexacarbonyl complexes作者:Elvira Montenegro、Albert Moyano、Miquel A Pericàs、Antoni Riera、Angel Alvarez-Larena、Joan-F PiniellaDOI:10.1016/s0957-4166(99)00032-4日期:1999.2afford 1,3-rearranged enantiomerically enriched α-butyl and α-phenyl enones. The absolute configuration of the Pauson–Khand adducts was determined by X-ray diffraction. The decreased enantiomeric excess of the final products uncovered an unprecedented low temperature racemization of the hexacarbonyl dicobalt complexes of alkynyl sulfoxides.
-
First formation of 1,1-dihalo-1,3-butadienes from reactions of dichloro- and dibromocarbenes with cyclopropenes via new addition-rearrangements作者:Jürgen Weber、Linxiao Xu、Udo H. BrinkerDOI:10.1016/s0040-4039(00)61306-0日期:1992.8dichloro-, dibromo-, and bromofluorocarbene, 2,3-diaryl-1,1-dihalo-1,3-butadienes are formed. In addition, 1,3-diaryl-2,3-dihalocyclo-1-butenes are formed. For the formation of the 1,1-dihalobutadienes, new addition-rearrangement mechanisms are proposed. Furthermore, some reactions of cyclobutenes with dihalocarbenes are described.
-
Cyclobutene photochemistry. Nonstereospecific photochemical ring opening of simple cyclobutenes作者:K. Brady Clark、William J. LeighDOI:10.1021/ja00254a030日期:1987.9The photochemistry of bicyclo(3.2.0)hept-6-ene, bicyclo(4.2.0)oct-7-ene, and cis- and trans-3,4-dimethylcyclobutene has been investigated in hydrocarbon solution with monochromatic far-ultraviolet (185 and 193 nm) light sources. All of these simple cyclobutene derivatives undergo ring opening to yield the isomeric 1,3-dienes, and the latter three open nonstereospecifically to yield mixtures of the研究了双环(3.2.0)庚-6-烯、双环(4.2.0)辛-7-烯和顺式和反式-3,4-二甲基环丁烯在具有单色远紫外光的烃溶液中的光化学( 185 和 193 nm) 光源。所有这些简单的环丁烯衍生物都进行开环以产生异构的 1,3-二烯,后三种以非立体有择的方式打开以产生可能的几何异构体的混合物。异构的 3,4-二甲基环丁烯产生三种 2,4-己二烯异构体的不同混合物,并且在每种情况下,混合物被加权以有利于轨道对称性禁止异构体。已尝试在纯反旋转的背景下分析双环 (4.2.0) 辛烯和异构 3,4-二甲基环丁烯开环的相对异构二烯产率,最近的 ab initio 计算表明的绝热开环机制应该是可能的。虽然前一种化合物的结果与这种机制一致,但对后两种化合物光解产生的异构 2,4-己二烯的相对产率的分析表明,通过正式禁止的旋转途径进行的光化学开环可能在一定程度上竞争具有旋转环开口。
表征谱图
-
氢谱1HNMR
-
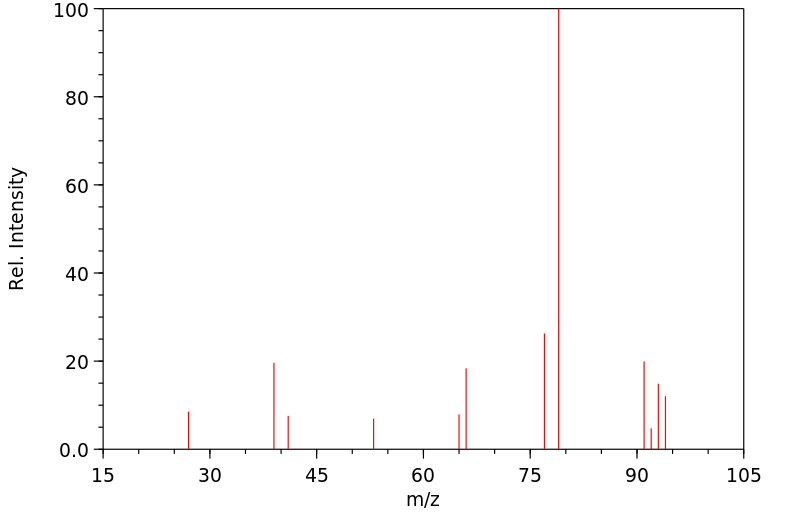
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷