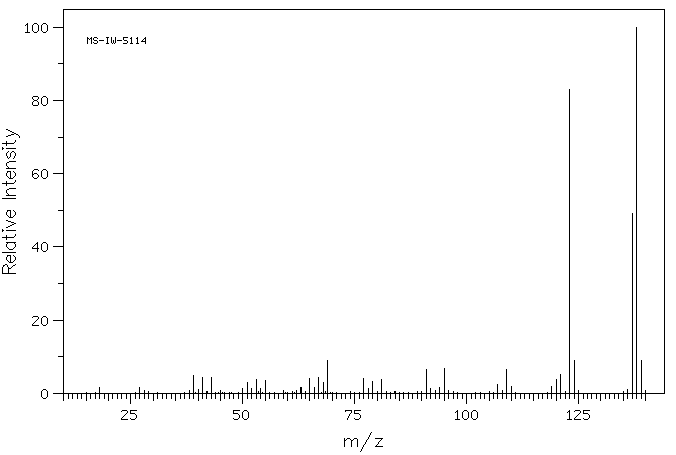
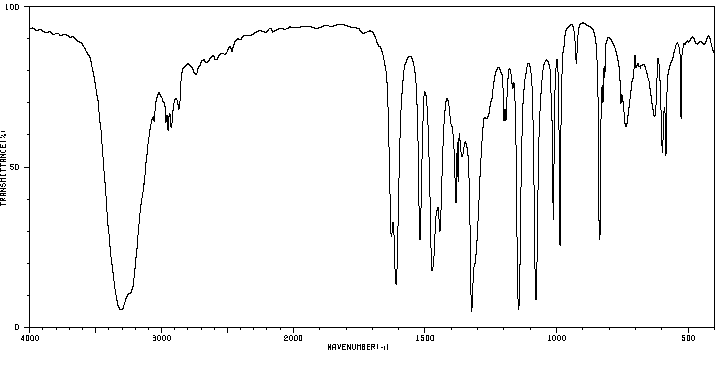
4,5-二甲基间苯二酚 | 527-55-9
中文名称
4,5-二甲基间苯二酚
中文别名
——
英文名称
4,5-dimethylresorcinol
英文别名
1,5-dihydroxy-3,4-dimethylbenzene;4,5-dimethylbenzene-1,3-diol
CAS
527-55-9
化学式
C8H10O2
mdl
MFCD00195568
分子量
138.166
InChiKey
RCNCKKACINZDOI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:135-137 C
-
沸点:213.57°C (rough estimate)
-
密度:1.1994
-
LogP:1.680 (est)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37
-
危险类别码:R36/37/38
SDS
| Name: | 4 5-Dimethylresorcinol Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 527-55-9 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 527-55-9 | 4,5-Dimethylresorcinol | ca. 100 | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.Light sensitive.Air sensitive.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation. Place under an inert atmosphere.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Store protected from light. Handle under an inert atmosphere. Store protected from air. Use only in a chemical fume hood. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container. Do not expose to air. Store protected from light. Store under an inert atmosphere.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 527-55-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: tan
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 284 deg C
Freezing/Melting Point: 136 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H10O2
Molecular Weight: 138.0688
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Air sensitive. Light sensitive.
Conditions to Avoid:
Incompatible materials, light, dust generation, exposure to air, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 527-55-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4,5-Dimethylresorcinol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 527-55-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 527-55-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 527-55-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-甲氧基-2,3-二甲基苯酚 2,3-Dimethyl-5-methoxyphenol 34883-01-7 C9H12O2 152.193 2,4-二羟基-6-甲基苯甲醛 2,4-dihydroxy-6-methylbenzaldehyde 487-69-4 C8H8O3 152.15 3,4-二甲基苯酚 3,4-Dimethylphenol 95-65-8 C8H10O 122.167 1,3-二甲氧基-5,6-二甲基苯 1,3-dimethoxy-5,6-dimethylbenzene 59968-26-2 C10H14O2 166.22 2-氯-4,5-二甲基-间苯二酚 4-Chlor-3,5-dihydroxy-o-xylol 17554-87-9 C8H9ClO2 172.611 —— 5,5'-dimethyl-4,4'-methanediyl-di-resorcinol 111666-02-5 C15H16O4 260.29 2,4-二甲氧基-6-甲基苯甲醛 2,4-dimethoxy-6-methyl-benzaldehyde 7149-90-8 C10H12O3 180.203 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4,5,6-三甲基-1,3-苯二酚 4,5,6-trimethyl-resorcinol 5700-67-4 C9H12O2 152.193 5-甲氧基-2,3-二甲基苯酚 2,3-Dimethyl-5-methoxyphenol 34883-01-7 C9H12O2 152.193 2,4-二羟基-6-甲基苯甲醛 2,4-dihydroxy-6-methylbenzaldehyde 487-69-4 C8H8O3 152.15 4,6-二羟基-2,3-二甲基苯甲醛 2,4-dihydroxy 5,6-dimethylbenzaldehyde 2990-31-0 C9H10O3 166.177
反应信息
-
作为反应物:描述:参考文献:名称:New heteroarotinoid compounds摘要:通式(I)的化合物:##STR1##其中:R表示氢原子、卤原子或羟基、低烷基、低烷氧基、羧基、(低烷氧基)羰基、(低芳基烷氧基)羰基、氨基羰基、(低烷基或双烷基)氨基羰基或(低芳基烷基)氨基羰基、带有杂环基团的N-取代氨基羰基,或硫基、(低烷基)硫基、磺酰基或(低烷基)磺酰基,R.sub.1、R.sub.2、R.sub.3和R.sub.4,可能相同也可能不同,表示氢原子、卤原子或低烷基、低烯基、低烷氧基或低烯氧基,可选地取代一个或多个卤原子,它们的异构体、对映体、非对映体异构体,以及当R表示羧基时,它们与药学上可接受的碱形成的加合盐,以及当R含有碱性基团时,它们与药学上可接受的酸形成的加合盐。公开号:US04975455A1
-
作为产物:参考文献:名称:间苯二酚的新途径摘要:的羟基化对位烷基化或2,6-二烷基过氧化氢的苯酚在SBF 5 -HF产量的间苯二酚,亲电与反应ö -protonated衬底。DOI:10.1039/c39800001128
文献信息
-
PROCESS FOR STRAIGHTENING KERATIN FIBRES WITH A HEATING MEANS AND DENATURING AGENTS申请人:Philippe Michel公开号:US20100028280A1公开(公告)日:2010-02-04The invention relates to a process for straightening keratin fibres, comprising: (i) a step in which a straightening composition containing at least two denaturing agents is applied to the keratin fibres, (ii) a step in which the temperature of the keratin fibres is raised, using a heating means, to a temperature of between 110 and 250° C.该发明涉及一种直发角蛋白纤维的拉直过程,包括:(i)将至少两种变性剂含有的拉直组合物涂抹到角蛋白纤维上的步骤,(ii)使用加热装置将角蛋白纤维的温度升高至110至250°C的步骤。
-
Synthesis of Tetra- and Pentasubstituted Benzenes from Dimedone and Derivatives作者:Peter H. Nelson、Janis T. NelsonDOI:10.1055/s-1992-26360日期:——Treatment of dimedone (5,5-dimethylcyclohexane-1,3-dione) and derivatives with one molar equivalent of sulfuric acid in trifluoroacetic anhydride leads, via sulfonation and a 1,2-methyl group migration, to a variety of dimethylresorcinol derivatives. The reaction has been performed on substrates bearing ester, alkoxy, halo and amino substituents to produce a variety of polysubstituted benzenes. Transient sulfonated intermediates were observed by NMR.
-
MODIFIER FOR AROMATIC POLYESTER AND AROMATIC POLYESTER RESIN COMPOSITION COMPRISING THE SAME申请人:TABATA Masayoshi公开号:US20110224343A1公开(公告)日:2011-09-15The present invention provides a modifier for aromatic polyesters which enhances the melt fluidity of aromatic polyesters without a significant decrease in the heat resistance of the aromatic polyesters, and an aromatic polyester resin composition including the modifier for aromatic polyesters. The present invention relates to a modifier for aromatic polyesters comprising polyhydric phenol residues and residues of aromatic polycarboxylic acid, acid halide or acid anhydride thereof, and the modifier comprises a material having a structure composed of a first residue selected from the group consisting of divalent residues represented by Formula (I): —Ar—W 1 x —Ar— and by Formula (II): —Ar—, the first residues being bonded to two identical or different second residues selected from the group consisting of monovalent residues represented by Formula (III): and monovalent residues represented by Formula (IV): —O—C(O)—R 7 —.
-
The biosynthesis of phenols—XVII作者:J.A. Ballantine、C.H. Hassall、B.D. JonesDOI:10.1016/s0031-9422(00)88601-x日期:1968.9Abstract Three metabolites which were isolated from different mutant strains of Aspergillus rugulosus have been identified as 2,4-dihydroxy-6-methylbenzaldehyde (III), 2,4-dihydroxy-6-(hydroxymethyl)-benzaldehyde (IV), and 3,3′-dihydroxy-5,5′-dimethyldiphenyl ether (V).摘要 从不同的皱曲霉突变菌株中分离出的三种代谢物已被鉴定为 2,4-二羟基-6-甲基苯甲醛 (III)、2,4-二羟基-6-(羟甲基)-苯甲醛 (IV) 和 3, 3'-二羟基-5,5'-二甲基二苯醚 (V)。
-
Biologically active 1,3-bis-aromatic-prop-2-en-1-ones, 1,3-bis-aromatic-propan-1-ones, and 1,3-bis-aromatic-prop-2-yn-1-ones申请人:Statens Serum Institute公开号:US20030065039A1公开(公告)日:2003-04-03The invention relates to the use of 1,3-bis-aromatic-prop-2-en-1-ones (chalcones), 1,3-bis-aromatic-propan-1-ones (dihydrochalcones), and 1,3-bis-aromatic-prop-2-yn-1-ones for the preparation of pharmaceutical compositions for the treatment or prophylaxis of a number of serious diseases including i) conditions relating to harmful effects of inflammatory cytokines, ii) conditions involving infection by Helicobacter species, iii) conditions involving infection by viruses, iv) neoplastic disorders, and v) conditions caused by microorganisms or parasites. The invention also relates to novel chalcones and dihydrochalcones (especially alkoxy substituted variants) having advantageous substitution patterns with respect to their effect as drug substances, and to methods of preparing them, as well as to pharmaceutical compositions comprising the novel chalcones. Moreover, the present invention relates to a method for the isolation of Leishmania fumarate reductase, QSAR methodologies for selecting potent compounds for the above-mentioned purposes.该发明涉及使用1,3-双芳基-丙-2-烯-1-酮(香豆素)、1,3-双芳基-丙酮-1-酮(二氢香豆素)和1,3-双芳基-丙-2-炔-1-酮制备用于治疗或预防多种严重疾病的药物组合物,包括i)与炎症细胞因子有害作用相关的疾病,ii)涉及幽门螺旋杆菌感染的疾病,iii)涉及病毒感染的疾病,iv)肿瘤性疾病,v)由微生物或寄生虫引起的疾病。该发明还涉及新型香豆素和二氢香豆素(特别是烷氧基取代变体),其具有有利的取代模式,对其作为药物物质的效果,以及制备它们的方法,以及包含新型香豆素的药物组合物。此外,本发明涉及一种用于分离利什曼氏弓形虫富马酸还原酶的方法,以及用于选择上述目的的有效化合物的QSAR方法。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚