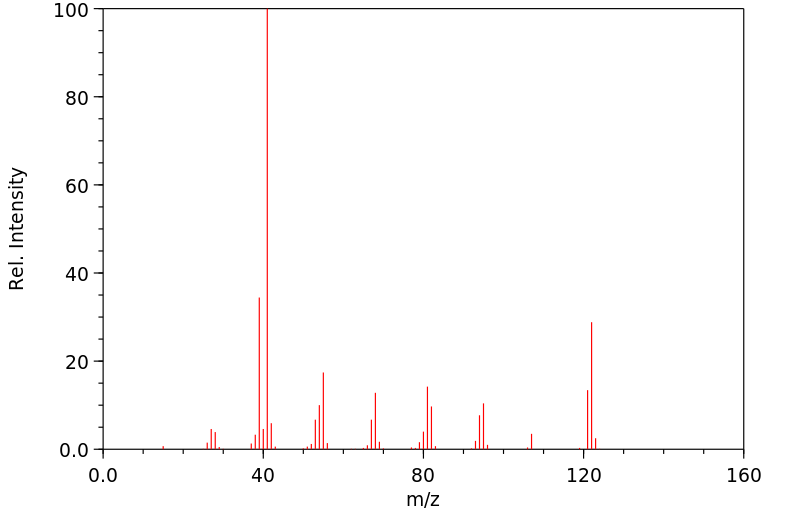
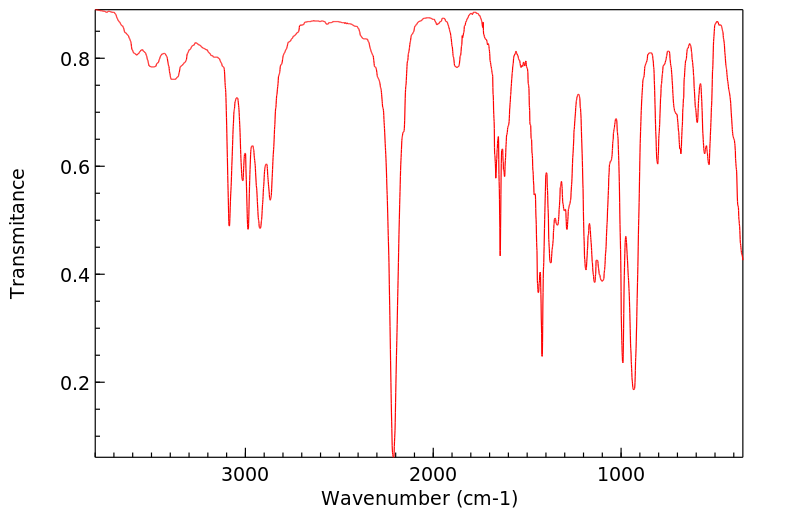
Phenyl chloro(thionoformate) reacts rapidly with unhindered tertiary aliphatic
amines at 20° to give a thiocarbamate and an alkyl chloride.
Dialkylcyclohexylamines react surprisingly rapidly to form predominantly
cyclohexene. The thiocarbamates are converted into the secondary amine salt by
treatment with dimethyl sulfate, followed by hydrolysis with water. Rates of
reaction and alkyl group cleavage selectivity in amines were found to be
superior or comparable to those previously reported with
chloroformates.
苯基
氯代(
硫代
甲酸酯)在20°下与无障碍的三级脂肪胺迅速反应,生成
硫代
氨酸酯和烷基
氯化物。二烷基
环己胺出奇地迅速反应,主要生成
环己烯。
硫代
氨酸酯经过甲基
硫酸二甲酯处理后,再用
水水解转化为二级胺盐。在
氨基中,反应速率和烷基团裂解选择性被发现优于或与以前报道的
氯代
甲酸酯相媲美。