(2,3,4,5,6-五氯苯基)2-氯乙酸酯 | 2948-20-1
中文名称
(2,3,4,5,6-五氯苯基)2-氯乙酸酯
中文别名
——
英文名称
2-chloroacetic acid (2,3,4,5,6-pentachlorophenyl) ester
英文别名
Chlor-essigsaeure-pentachlorphenylester;Pentachlorphenyl-chloracetat;Pentachlorphenyl-monochloracetat;chloro-acetic acid pentachlorophenyl ester;Pentachlorophenyl chloroacetate;(2,3,4,5,6-pentachlorophenyl) 2-chloroacetate
CAS
2948-20-1
化学式
C8H2Cl6O2
mdl
——
分子量
342.821
InChiKey
OCCPWFCBTICIIN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.4
-
重原子数:16
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Petrina,N.B. et al., Journal of general chemistry of the USSR, 1977, vol. 47, p. 946 - 949摘要:DOI:
-
作为产物:描述:参考文献:名称:Kamennov,N.A. et al., Journal of applied chemistry of the USSR, 1967, vol. 40, # 3, p. 624 - 627摘要:DOI:
文献信息
-
Cyclic peptide models of the Ca2+-binding loop of α-lactalbumin作者:Viktor Farkas、Elemér Vass、Ignace Hanssens、Zsuzsa Majer、Miklós HollósiDOI:10.1016/j.bmc.2005.06.040日期:2005.9coordinating the cation. The structural features of synthetic peptides and their Ca2+-binding properties were investigated in solution by circular dichroism (CD) and Fourier transform infrared (FTIR) spectroscopy. In water, the CD curves show a strong negative band below 200 nm as a sign of the presence of unfolded conformers. In TFE, all cyclic peptides were found to have a CD spectrum, reflecting the设计并合成了一系列具有不同接头的环状肽,以模拟α-乳清蛋白(LA)的肘型Ca2 +结合环。Ca2 +结合环的所有氨基酸在不同物种的LA之间都具有非常好的保守性,序列为Lys79-Phe-Leu-Asp82-Asp-Asp-Leu-Thr-Asp87-Asp88,其中Asp82,Asp87和Asp88和Lys79和Asp84的酰胺羰基氧原子参与Ca2 +的结合。还制备了含丙氨酸的模型以监测结合(82、87-88)和非结合Asp残基(83-84)在协调阳离子中的作用。通过溶液的圆二色性(CD)和傅立叶变换红外光谱(FTIR)研究了合成肽的结构特征及其与Ca 2+的结合特性。在水里,CD曲线显示在200 nm以下有很强的负带,表明存在未折叠构象异构体。在TFE中,发现所有环状肽均具有CD光谱,反映了折叠(转向)构象体的存在。Ca2 +的作用取决于模型的结构和浓度以及Ca2 +与肽的比率(r(cat))。观察到了含ALA肽的Ca2
-
Development of Cyclic NGR Peptides with Thioether Linkage: Structure and Dynamics Determining Deamidation and Bioactivity作者:Kata Nóra Enyedi、András Czajlik、Krisztina Knapp、András Láng、Zsuzsa Majer、Eszter Lajkó、László Kőhidai、András Perczel、Gábor MezőDOI:10.1021/jm501630j日期:2015.2.26interest, in particular due to their potential applications in drug targeting. Here we report the synthesis and structural analysis of novel thioether bond-linked cyclic NGR peptides. Our results show that their chemostability (resistance against spontaneous decomposition forming isoAsp and Asp derivatives) strongly depends on both sample handling conditions and structural properties. A significant correlation识别肿瘤新脉管系统中CD13受体的NGR肽备受关注,特别是由于其在药物靶向中的潜在应用。在这里我们报告了新型硫醚键连接的环状NGR肽的合成和结构分析。我们的结果表明,它们的化学稳定性(抵抗形成异Asp和Asp衍生物的自发分解的能力)在很大程度上取决于样品处理条件和结构性质。发现化学稳定性与结构度量之间存在显着相关性,例如NH Gly –CO Asn-sc距离。Asn的侧链取向是关键的决定因素。如果它远离HN Gly,化学稳定性增加。结构稳定因子(例如,氢键)降低了它们的内部动力学,因此生物分子对自发分解的抵抗力甚至更高。在A2058黑色素瘤细胞系中检查了环状NGR肽对细胞粘附的影响。发现一些研究的肽具有长期特征逐渐增加了细胞粘附,表明负责粘附诱导作用的整联蛋白结合iso Asp衍生物的时间依赖性形成。
-
Novel substituted 2,3-benzodiazepine derivatives申请人:Solyom Sandor公开号:US20070027143A1公开(公告)日:2007-02-01The invention relates to new 2,3-benzodiazepine derivatives of formula (I), isomers and acid addition salts thereof and to pharmaceutical compositions containing the same, as well as to pharmaceutical compositions and methods of using the same suitable for treating conditions associated with muscle spasms, epilepsy, acute and chronic forms of neurodegenerative diseases as well as preventing, treating or alleviating the symptoms of acute and chronic inflammatory disorders.本发明涉及公式(I)的新型2,3-苯二氮平衍生物、其异构体和酸加成盐,以及含有它们的制药组合物,以及适用于治疗与肌肉痉挛、癫痫、急性和慢性神经退行性疾病以及预防、治疗或缓解急性和慢性炎症性疾病症状的制药组合物和使用方法。
-
Bioconjugates comprising Aß-autoantibody-specific epitopes for active immunotherapy and diagnosis of Alzheimer's disease申请人:Universität Konstanz公开号:EP2143447A1公开(公告)日:2010-01-13Bioconjugates are provided comprising a scaffold and an antigen, wherein the antigen is a peptide comprising a region from the ß-amyloid peptide (Aß). Such bioconjugates are useful for immunotherapy and diagnosis of Alzheimer's disease.
-
Boccacci; Bettini, Rendiconti - Istituto superiore di sanita, 1956, vol. 19, p. 1237,1239作者:Boccacci、BettiniDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
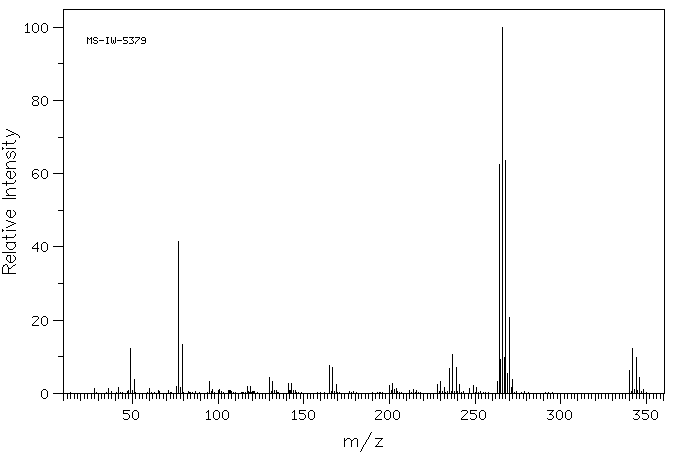
质谱MS
-
碳谱13CNMR
-
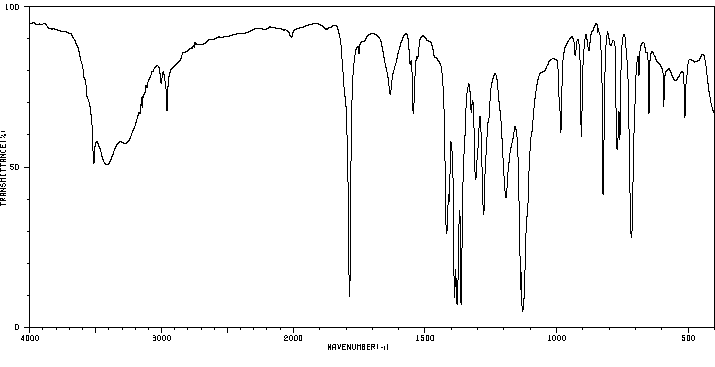
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰亚胺四聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺六聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺-酰胺-PEG8-四氟苯酚酯
马来酰亚胺-四聚乙二醇-五氟苯酯
马来酰亚胺-三聚乙二醇-五氟苯酚酯
靛酚乙酸酯
阿立哌唑标准品002
间硝基苯基戊酸酯
间氯苯乙酸乙酯
间乙酰苯甲酸
钾4-乙酰氧基苯磺酸酯
酚醛乙酸酯
邻苯二酚二乙酸酯
邻甲苯基环己甲酸酯
邻甲氧基苯乙酸酯
辛酸苯酯
辛酸对甲苯酚酯
辛酸五氯苯基酯
辛酸-(3-氯-苯基酯)
辛酰溴苯腈
苯酰胺,3,4-二(乙酰氧基)-N-[6-氨基-1,2,3,4-四氢-1-(4-甲氧苯基)-3-甲基-2,4-二羰基-5-嘧啶基]-
苯酚-乳酸
苯酚,4-异氰基-,乙酸酯(ester)
苯酚,4-[(四氢-2H-吡喃-2-基)氧代]-,乙酸酯
苯酚,3-(1,1-二甲基乙基)-,乙酸酯
苯酚,2-溴-3-(二溴甲基)-5-甲氧基-,乙酸酯
苯甲醇,4-(乙酰氧基)-3,5-二甲氧基-
苯甲酸,4-(乙酰氧基)-2-氟-
苯氧基氯乙酸苯酯
苯基金刚烷-1-羧酸酯
苯基氰基甲酸酯
苯基庚酸酯
苯基庚-6-炔酸酯
苯基己酸酯
苯基呋喃-2-羧酸酯
苯基吡啶-2-羧酸酯
苯基十一碳-10-烯酸酯
苯基乙醛酸酯
苯基乙酸酯-d5
苯基丙二酸单苯酯
苯基丙-2-炔酸酯
苯基丁-2,3-二烯酸酯
苯基4-乙基环己烷羧酸
苯基3-乙氧基-3-亚氨基丙酸盐
苯基2-(苯磺酰基)乙酸酯
苯基2-(4-甲氧基苯基)乙酸酯
苯基2-(2-甲氧基苯基)乙酸酯
苯基2-(2-甲基苯基)乙酸酯
苯基-乙酸-(2-甲酰基-苯基酯)
苯基-乙酸-(2-环己基-苯基酯)