1,2-bis(2-benzimidazolyl)ethane | 3575-07-3
中文名称
——
中文别名
——
英文名称
1,2-bis(2-benzimidazolyl)ethane
英文别名
1,2-bis(benzimidazol-2-yl)ethane;1,2-bis(1H-benzo[d]imidazol-2-yl)ethane;2-[2-(1H-Benzimidazol-2-yl)ethyl]-1H-benzimidazole
CAS
3575-07-3
化学式
C16H14N4
mdl
MFCD00087492
分子量
262.314
InChiKey
IXEHZMUOBBZTNR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:360 °C
-
沸点:626.1±38.0 °C(Predicted)
-
密度:1.350±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:20
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:57.4
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
海关编码:2933990090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— trans-1,2-di(1H-benzo[d]imidazol-2-yl)ethene 24226-82-2 C16H12N4 260.298 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2-bis(5-amino-2-benzimidazolyl)ethane 41607-49-2 C16H16N6 292.343 —— trans-1,2-di(1H-benzo[d]imidazol-2-yl)ethene 24226-82-2 C16H12N4 260.298 —— 1,2-bis(N-methylbenzimidazol-2-yl)ethane 4508-05-8 C18H18N4 290.368 —— 1H-Benzimidazole, 2,2'-(1,2-ethanediyl)bis[5-nitro- 5887-57-0 C16H12N6O4 352.309
反应信息
-
作为反应物:描述:参考文献:名称:Supramolecular interactions of bisbenzimidazolyl derivatives with cucurbit[7]uril, potential axle molecules bearing a novel fluorescent signal response摘要:The supramolecular properties of cucurbit[7]uril and 1,omega-bisbenzimidazolyl derivatives have been studied by H-1 NMR, fluorescence emission spectroscopy, and X-ray single crystal analysis. The distinctly fluorescent response behaviors indicated that the benzimidazole moiety here not only acts as the optical signaling unit for the pseudorotaxane system, but also behaves as a proton acceptor during the host-guest complexation. Furthermore, the fluorescent signals intensity could be adjusted by varying the alkylene spacer between the two benzimidazole moieties. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2013.05.031
-
作为产物:描述:参考文献:名称:Phillips, Journal of the Chemical Society, 1928, p. 2395摘要:DOI:
文献信息
-
Expedient synthesis of benzimidazoles using amides作者:Pramod P. Kattimani、Ravindra R. Kamble、Gangadhar Y. MetiDOI:10.1039/c5ra00021a日期:——
In the present report an efficient, rapid, facile and inexpensive route for the synthesis of benzimidazoles using 1,2-arylenediamines and amides in acidic medium under thermal/microwave conditions is developed.
在本报告中,开发了一种高效、快速、简便且廉价的合成苯并咪唑的途径,该途径利用酸性介质下的热/微波条件使用1,2-芳二胺和酰胺进行合成。 -
Structure, DNA minor groove binding, and base pair specificity of alkyl- and aryl-linked bis(amidinobenzimidazoles) and bis(amidinoindoles)作者:Terri A. Fairley、Richard R. Tidwell、Isaac Donkor、Noreen A. Naiman、Kwasi A. Ohemeng、Richard J. Lombardy、James A. Bentley、Michael CoryDOI:10.1021/jm00064a008日期:1993.6A series of bis(amidinobenzimidazoles) and bis(amidinoindoles) with varied linking chains connecting the aromatic groups and various modifications to the basic amidino groups have been prepared. The calf thymus (CT) DNA and nucleic acid homopolymer [poly(dA).poly(dT),poly(dA-dT).poly-(dA-dT), and poly(dG-dC).poly(dG-dC)] binding properties of these compounds have been studied by thermal denaturation已经制备了一系列具有变化的连接链的双(ami基苯并咪唑)和双(ami基吲哚),所述连接链连接芳族基团并且对碱性a基进行了各种修饰。小牛胸腺(CT)DNA和核酸均聚物[poly(dA).poly(dT),poly(dA-dT).poly-(dA-dT)和poly(dG-dC).poly(dG-dC) )]已经通过热变性(ΔTm)和粘度研究了这些化合物的结合性能。该化合物对poly(dA).poly(dT)和poly(dA-dT).poly(dA-dT)的亲和力大于对poly(dG-dC).poly(dG-dC)的亲和力。粘度滴定表明该化合物不通过嵌入结合。分子模型研究和生物物理数据表明,分子与CT DNA和均聚物的小沟结合。分子形状的分析与这种核酸结合模式一致。与苯并咪唑环相连的亚甲基数量为偶数的化合物比亚甲基数量为奇数的化合物对DNA的亲和力更高。确定分子中四个定义的基团的曲率半径的分子模
-
Boric Acid-Catalyzed Direct Condensation of Carboxylic Acids with Benzene-1,2-diamine into Benzimidazoles作者:Nenad Maraš、Marijan KočevarDOI:10.1002/hlca.201100064日期:2011.10applicability of boric acid catalysis for the direct condensation of carboxylic acids with benzene‐1,2‐diamine to give 2‐substituted benzimidazoles was investigated. It was found that catalytic amounts (5–10 mol‐%) of boric acid efficiently promote the cyclocondensation of aliphatic carboxylic acids in refluxing toluene. In addition, the relatively neutral conditions allow the use of acid‐sensitive substrates
-
Bisbenzimidazole Derivatives as Potential Antimicrobial Agents: Design, Synthesis, Biological Evaluation and Pharmacophore Analysis作者:Ronak Haj Ersan、Kayhan Bolelli、Serpil Gonca、Aylin Dogen、Serdar Burmaoglu、Oztekin AlgulDOI:10.1007/s11094-021-02389-x日期:2021.5In an attempt to design and synthesize a potent class of antimicrobials, 1,2-phenylenediamine derivatives were reacted with various aliphatic and heteroaliphatic dicarboxylic acids to generate a small library of 26 head-to-head bisbenzimidazole compounds (16 – 42) using the polyphosphoric acid method. These compounds were screened for their antibacterial activity and their antifungal activity. Compound 25 showed maximum potency against both Gram-positive and Gram-negative bacterial strains with minimum inhibitory concentration (MIC) values in the range of 7.81 – 31.25 μg/mL. In particular, it showed the maximum MIC values of 7.81 μg/mL against Gram-negative bacteria, which was four-fold more active than the standard drug ampicillin (MIC = 32.25 μg/mL). Compound 19 was found to be the most active against S. aureus with a MIC value of < 3.90 μg/mL, whereas the remaining compounds showed only low-to-moderate activity. Furthermore, all compounds exhibited low activity against all fungal strains in comparison to the standard drug fluconazole. I addition, pharmacophore hypotheses were generated to analyze structure–activity relationships between the molecular structures and antimicrobial activities on E. coli. This pharmacophore model can be useful in order to design new antimicrobial drugs. It can be suggested that the substitution of a phenyl ring at the 5/6 and 5′/6′ positions in symmetric bisbenzimidazole derivatives produces compounds with promising antimicrobial activity.为了设计并合成一类高效的抗菌药物,我们通过多磷酸法,将1,2-苯二胺衍生物与各种脂肪族和杂脂肪族二羧酸反应,生成了一个包含26个头对头双苯并咪唑化合物(16 – 42)的小型库。这些化合物经过筛选,评估了它们的抗菌活性和抗真菌活性。化合物25对革兰氏阳性和革兰氏阴性细菌株显示出最高的活性,其最低抑制浓度(MIC)值在7.81至31.25 μg/mL范围内。特别是,它对革兰氏阴性细菌显示出最高的MIC值7.81 μg/mL,比标准药物氨苄西林(MIC = 32.25 μg/mL)高出四倍。化合物19对金黄色葡萄球菌显示出最高的活性,其MIC值小于3.90 μg/mL,而其他化合物仅显示出低至中等的活性。此外,与标准药物氟康唑相比,所有化合物对所有真菌株均显示出低活性。此外,生成了药效团假设,以分析大肠杆菌上的分子结构与抗菌活性之间的结构-活性关系。这个药效团模型对于设计新的抗菌药物可能非常有用。可以建议,在对称双苯并咪唑衍生物的5/6和5′/6′位置上取代苯环会产生具有有前景的抗菌活性的化合物。
-
Ti (IV) Complexes of Some Heterocyclic Ligands Synthesis, Characterization and Ethylene Polymerization Activity作者:Hamdi ElagabDOI:10.13005/ojc/320177日期:2016.3.2531 complexes of bis - (benzimidazole, benzothiazole and benzoxazole) compounds with Ti (IV) metal centers were synthesized, characterized, activated with methylalumoxane (MAO) and then tested for catalytic ethylene polymerization. The activities of the various catalysts were found to be functions of the hetero atoms in the ligand frameworks. The highest activity was obtained with 39 / MAO (573 kg PE / mol cat. h). The produced polyethylenes showed high molecular weights (up to 1.5 ×106 g/mol) and broad molecular weight distributions (PD = 65). This could result from different interactions of the MAO counterion with the heteroatoms of the catalyst ligand generating different active sites.
表征谱图
-
氢谱1HNMR
-
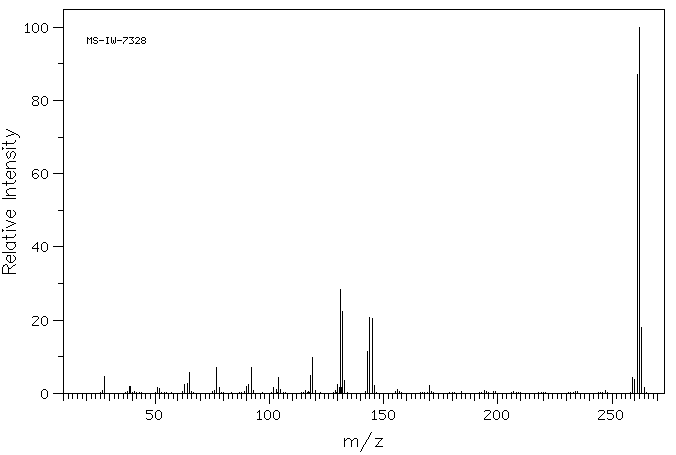
质谱MS
-
碳谱13CNMR
-
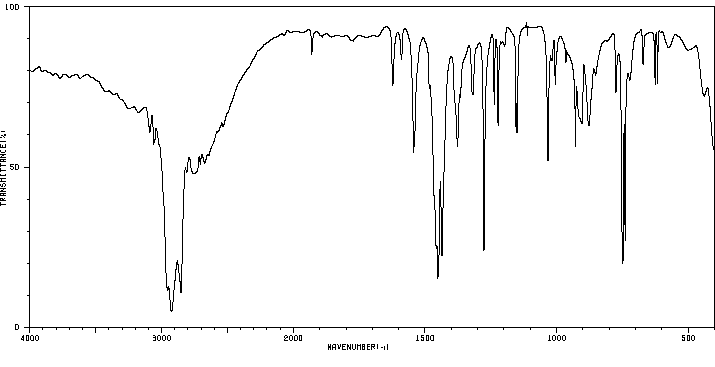
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
麦穗宁
马哌斯汀
颜料橙62
顺式-5,6-二氢-4,5-二甲基-4H-咪唑并[1,5,4-De]喹喔啉
韦罗肟
青菌灵
雷贝拉唑钠
雷贝拉唑硫醚N-氧化物
雷贝拉唑砜 N-氧化物
雷贝拉唑砜
雷贝拉唑杂质2
雷贝拉唑 N-氧化物
雷贝拉唑
阿苯达唑砜
阿苯达唑杂质L
阿苯达唑杂质J(EP)
阿苯达唑杂质J
阿苯达唑杂质F
阿苯达唑杂质14
阿苯达唑杂质13
阿苯达唑亚砜
阿苯达唑
阿苯哒唑砜-D3
阿苯哒唑-D3
阿地本旦
阿司咪唑-d3
阿司咪唑
钠4-[5-氯-2-[(E,3E)-3-[6-氯-1-乙基-3-(4-磺酸丁基)-5-(三氟甲基)苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-6-(三氟甲基)苯并咪唑-1-鎓-1-基]丁烷-1-磺酸盐
邻甲磺酰胺基苯乙酸
那地特罗
达比加群酯杂质M
达比加群酯杂质4
达比加群酯杂质1
达比加群酯杂质
达比加群酯N-氧化物
达比加群酯
达比加群脂杂质10
达比加群甲酯杂质
达比加群杂质J
达比加群杂质J
达比加群杂质F
达比加群杂质E
达比加群杂质D
达比加群杂质C5
达比加群杂质38
达比加群杂质13
达比加群杂质10(DABRC-10)
达比加群杂质10