2-(4-甲氧基苯氧基)乙酸甲酯 | 79704-02-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:50 °C
-
沸点:161 °C(Press: 10 Torr)
-
密度:1.128±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:14
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对甲氧基苯氧乙酸 2-(4-methoxyphenoxy)acetic acid 1877-75-4 C9H10O4 182.176 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 对甲氧基苯氧乙酸 2-(4-methoxyphenoxy)acetic acid 1877-75-4 C9H10O4 182.176 —— benzyl (4-methoxyphenoxy)acetate 500348-96-9 C16H16O4 272.301 (4-甲氧基-苯氧基)-乙酰氯 p-methoxyphenoxyacetyl chloride 42082-29-1 C9H9ClO3 200.622 —— 2-(4-methoxyphenoxy)acetohydroxamic acid 15267-80-8 C9H11NO4 197.191 (4-甲氧基苯氧基)-乙酸肼 (4-methoxyphenoxy)acetic acid hydrazide 21953-91-3 C9H12N2O3 196.206
反应信息
-
作为反应物:描述:参考文献:名称:无催化剂光氧化还原加成环化:利用芳基乙酸和马来酰亚胺之间的天然协同作用摘要:当在马来酰亚胺存在下用 UVA 照射时,适当官能化的羧酸会发生以前未知的光氧化还原反应。发现马来酰亚胺协同作用作为产生自由基的光氧化剂和作为自由基受体,不需要外在的光氧化还原催化剂。在 13 次制备性光解中获得了中等至极好的产率的产物色基吡咯二酮、硫代色基吡咯二酮和吡咯并喹啉二酮衍生物。原位核磁共振光谱用于研究每个反应。监测反应物衰变和产物堆积,从而绘制反应曲线。一种似是而非的机制,即光激发的马来酰亚胺充当氧化剂以产生自由基离子对,已被假设并得到 UV/Vis 的支持。光谱和 DFT 计算。DOI:10.1002/chem.201304929
-
作为产物:描述:参考文献:名称:Hayashi, Masatoshi; Wada, Kojiro; Munakata, Katsura, Agricultural and Biological Chemistry, 1983, vol. 47, # 11, p. 2653 - 2656摘要:DOI:
文献信息
-
α-Ketoheterocycles Able to Inhibit the Generation of Prostaglandin E2 (PGE2) in Rat Mesangial Cells作者:Anastasia Psarra、Maria A. Theodoropoulou、Martin Erhardt、Marina Mertiri、Christiana Mantzourani、Sofia Vasilakaki、Victoria Magrioti、Andrea Huwiler、George KokotosDOI:10.3390/biom11020275日期:——
Prostaglandin E2 (PGE2) is a key mediator of inflammation, and consequently huge efforts have been devoted to the development of novel agents able to regulate its formation. In this work, we present the synthesis of various α-ketoheterocycles and a study of their ability to inhibit the formation of PGE2 at a cellular level. A series of α-ketobenzothiazoles, α-ketobenzoxazoles, α-ketobenzimidazoles, and α-keto-1,2,4-oxadiazoles were synthesized and chemically characterized. Evaluation of their ability to suppress the generation of PGE2 in interleukin-1β plus forskolin-stimulated mesangial cells led to the identification of one α-ketobenzothiazole (GK181) and one α-ketobenzoxazole (GK491), which are able to suppress the PGE2 generation at a nanomolar level.
-
Efficient Synthetic Approach to Substituted Benzo[<i>b</i>]furans and Benzo[<i>b</i>]thiophenes by Iodine-Promoted Cyclization of Enaminones作者:Ehecatl Labarrios、Alberto Jerezano、Fabiola Jiménez、María del Carmen Cruz、Francisco Delgado、L. Gerardo Zepeda、Joaquín TamarizDOI:10.1002/jhet.1686日期:2014.7An efficient synthetic approach to the substituted benzo[b]furan and benzo[b]thiophene scaffolds by iodine‐mediated cyclization of the corresponding enaminones is described. This protocol was applied to a large series of these latter precursors to afford the respective benzoheterocycles substituted at the C‐2 position by a carbonyl group functionality. A study of the factors that control this process
-
Metal‐Free Twofold Electrochemical C−H Amination of Activated Arenes: Application to Medicinally Relevant Precursor Synthesis作者:Lars J. Wesenberg、Erika Diehl、Till J. B. Zähringer、Carolin Dörr、Dieter Schollmeyer、Akihiro Shimizu、Jun‐ichi Yoshida、Ute A. Hellmich、Siegfried R. WaldvogelDOI:10.1002/chem.202003852日期:2020.12.23materials suffers from wasteful or toxic precursors for the synthesis. In particular, the aromatic non‐protected primary amine function represents a versatile synthetic precursor, but its synthesis typically requires toxic oxidizing agents and transition metal catalysts. The twofold electrochemical amination of activated benzene derivatives via Zincke intermediates provides an alternative sustainable
-
Aza-Michael Addition of Acrylonitrile with 2-Aryloxymethylbenzimidazole Derivatives under Microwave Irradiation作者:Tai-Bao Wei、Mao-Tang Hua、Hai-Xiong Shi、Yong Liu、You-Ming ZhangDOI:10.3184/030823410x12798039968476日期:2010.8
A simple, rapid, and highly efficient method has been developed for the aza-Michael addition of acrylonitrile to 2-aryl-oxymethylbenzimidazole derivatives in the presence of anhydrous potassium carbonate under microwave irradiation. A series novel of 1-cyanoethyl-2-aryloxymethylbenzimidazole derivatives have been prepared and characterised by 1H NMR, 13C NMR, IR spectra and elemental analysis.
-
Design and Synthesis of Novel 4-Hydroxyl-3-(2-phenoxyacetyl)-pyran-2-one Derivatives for Use as Herbicides and Evaluation of Their Mode of Action作者:Kang Lei、Pan Li、Xue-Fang Yang、Shi-Ben Wang、Xue-Kun Wang、Xue-Wen Hua、Bin Sun、Lu-Sha Ji、Xiao-Hua XuDOI:10.1021/acs.jafc.9b03109日期:2019.9.18its great potential as a herbicide. More importantly, studying the molecular mode of action of compound II15 revealed that the novel triketone structure is a proherbicide of its corresponding phenoxyacetic acid auxin herbicide, which has a herbicidal mechanism similar to that of 2,4-D. The present work indicates that the 4-hydroxyl-3-(2-phenoxyacetyl)-pyran-2-one motif may be a potential lead structure为了开发含有β-三酮基序的新型除草剂,设计并合成了一系列4-羟基-3-(2-苯氧基乙酰基)-吡喃-2-酮衍生物。生物测定结果表明,即使剂量为187.5 g ha –1,化合物II15也具有良好的芽前除草活性。此外,与商业除草剂2,4-二氯苯氧基乙酸(2,4-D)相比,化合物II15的杂草控制范围更广,并且对小麦和玉米具有良好的农作物安全性。当以375 g ha –1施用时在出苗前的条件下,表明其作为除草剂的巨大潜力。更重要的是,研究化合物II15的分子作用方式表明,新的三酮结构是其相应的苯氧乙酸生长素除草剂的除草剂,其除草机理与2,4-D相似。目前的工作表明4-羟基-3-(2-苯氧基乙酰基)-吡喃-2-酮基序可能是潜在的铅结构,用于进一步开发新型的生长素型除草剂。
表征谱图
-
氢谱1HNMR
-
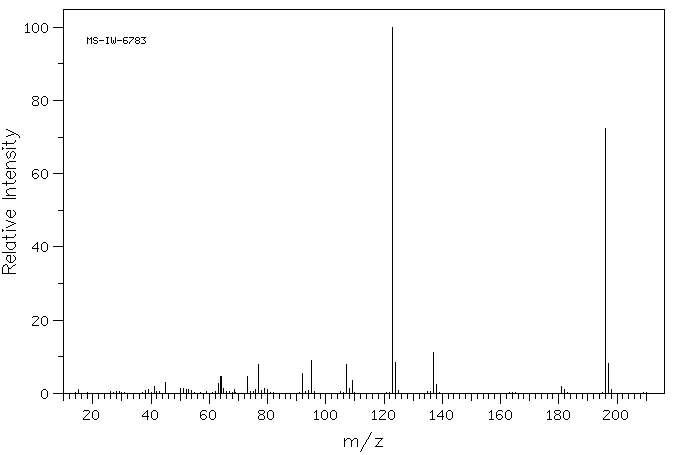
质谱MS
-
碳谱13CNMR
-
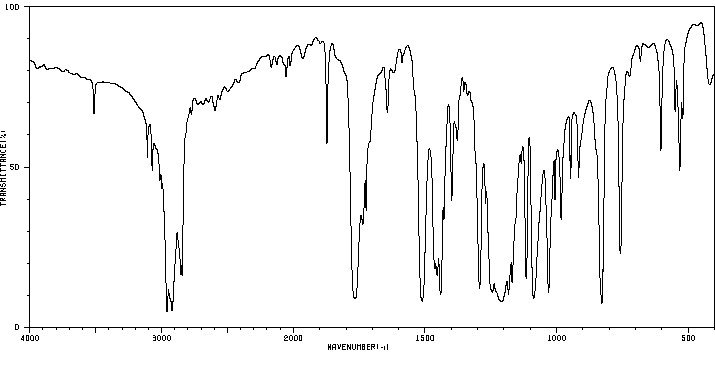
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息