2-(p-N,N-二甲基氨基苯基)-1H-苯并咪唑 | 2562-71-2
中文名称
2-(p-N,N-二甲基氨基苯基)-1H-苯并咪唑
中文别名
——
英文名称
2-(4-dimethylaminophenyl)-1H-benzimidazole
英文别名
4-(1H-benzo[d]imidazol-2-yl)-N,N-dimethylaniline;4-(1H-benzimidazol-2-yl)-N,N-dimethylaniline;2-(4-N,N-dimethylaminophenyl)-1H-benzimidazole;2-(4'-(N,N-dimethylamino)phenyl)benzimidazole;2-(4-dimethylaminophenyl)benzimidazole;2-<4-Dimethylamino-phenyl>-benzimidazol;2-(p-N,N-Dimethylaminophenyl)-1H-benzoimidazole
CAS
2562-71-2
化学式
C15H15N3
mdl
MFCD00441571
分子量
237.304
InChiKey
ZKBBGUJBGLTNEK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:18
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.133
-
拓扑面积:31.9
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2933290090
-
储存条件:室温
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : N-[4-(1H-Benzimidazol-2-yl)phenyl]-N,N-
dimethylamine
CAS-No. : 2562-71-2
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C15H15N3
Molecular Weight : 237,31 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 3,739
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation
May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methyl-2-(4-N,N-dimethylaminophenyl)benzimidazole 2562-72-3 C16H17N3 251.331
反应信息
-
作为反应物:描述:2-(p-N,N-二甲基氨基苯基)-1H-苯并咪唑 在 碘苯二乙酸 、 碘 、 palladium diacetate 作用下, 以 乙腈 为溶剂, 反应 3.0h, 以78%的产率得到4-(1H-benzo[d]imidazol-2-yl)-N,N-dimethyl-3-nitroaniline参考文献:名称:高价碘促进邻位多样化:以2-芳基苯并咪唑,喹唑啉和咪唑并吡啶为指导模板。摘要:使用高价碘作为关键试剂,报道了温和而有效的钯催化的不含(NH)2取代的苯并咪唑,喹唑啉和咪唑并吡啶的邻位C(sp2)-H多样化。在有氧条件下,只需在无机添加剂(Cs2CO3,I2,NaNO2)和高价碘试剂(二乙酰氧基碘)苯(PIDA)存在下简单地改变反应条件,即可将乙酰氧基,芳基,碘化物和硝基官能团引入同一底物。NaNO2与PIDA的组合已成功用于Pd催化的CH键硝化反应,从而获得了硝化的1,3 N-杂环文库。这种通用的邻位C(sp2)-H活化策略具有操作简便,反应时间短和底物丰富的可能性,不需要配体或银盐作为添加剂,DOI:10.1039/c9ob02533b
-
作为产物:描述:参考文献:名称:Aqueous Synthesis of 1-H-2-Substituted Benzimidazoles via Transition-Metal-Free Intramolecular Amination of Aryl Iodides摘要:通过碳氮交叉偶联反应合成苯并咪唑环系统的直接方法已经开发出来。在 2.0 等量的 K2CO3 存在下,于 100 °C 的水中反应 30 小时,N-(2-碘芳基)苯甲脒的分子内环化反应可提供中等至高产率的苯并咪唑衍生物。值得注意的是,该过程完全在水中进行,不需要使用任何额外的试剂/催化剂,因此从环保和经济的角度来看,该方法都具有很高的价值。DOI:10.3390/molecules171112506
文献信息
-
Nano-Ni(II)/Y Zeolite Catalyzed Synthesis of 2-Aryl- and 2-Alkyl Benzimidazoles Under Solvent-Free Conditions作者:A. Mobinikhaledi、M. Zendehdel、F. Goudarzi、G. Rezanejade BardajeeDOI:10.1080/15533174.2015.1137020日期:2016.10.2Synthesis of some benzimidazole derivatives via condensation reaction of o-phenylenediamine derivatives with aromatic aldehydes or orthoesters under solvent-free conditions over nano-Ni(II)/Y zeolite as a heterogeneous catalyst is reported. Some advantages of this green method are easy purification, environmentally friendly conditions, low catalyst loadings, and nontoxic nature.
-
Excited-State Proton and Charge Transfer in Protonated Amino and Methylated Derivatives of 2-(2′-Hydroxyphenyl)benzimidazole作者:Sonia Ríos Vázquez、J. Luis Pérez Lustres、Flor Rodríguez-Prieto、Manuel Mosquera、M. Carmen Ríos RodríguezDOI:10.1021/jp507917u日期:2015.2.12solvents, using steady-state and time-resolved fluorescence spectroscopy. The species investigated were 2-(4′-amino-2′-hydroxyphenyl)benzimidazole (1), the diethylamino analogue 2-(4′-N,N-diethylamino-2′-hydroxyphenyl)benzimidazole (2) and its N-methylated derivative 1-methyl-2-(4′-N,N-diethylamino-2′-hydroxyphenyl)benzimidazole (3). The O-methoxy derivatives of 2 and 3 (2-OMe and 3-OMe), and the simpler我们使用稳态和时间分辨荧光光谱研究了2-苯基苯并咪唑在不同溶剂中的单质子化和双质子化衍生物的激发态行为。种研究是2-(4'-氨基-2'-羟基苯基)苯并咪唑(1)中,二乙基氨基类似物2-(4'- Ñ,ñ -二乙基氨基-2'-羟基苯基)苯并咪唑(2)和它的ñ -甲基化的衍生物1-甲基-2-(4'- N,N-二乙氨基-2'-羟基苯基)苯并咪唑(3)。2和3(2-OMe和3-OMe的O-甲氧基衍生物),并研究了更简单的模型2-苯基苯并咪唑(4)及其4'-氨基(5)和4'-二甲基氨基(6)衍生物。我们发现的双阳离子1,2,和3(在苯并咪唑N3和在氨基质子化的)为强光酸,将其在羟基激发时去质子化在水溶液中(完全为2和3)给出基态单官能团的互变异构体。相反,未观察到这些物种的单阳离子发生光解离。取而代之的是,研究的一些单阳离子表现为分子转子,为此电子激发导致扭曲的分子内电荷转移(TICT)状态。的单阳离子2,3,2-OME,3-
-
Diversity-oriented synthesis of imidazo[2,1-<i>a</i>]isoquinolines作者:Shaoyu Mai、Yixin Luo、Xianyun Huang、Zhenghao Shu、Bingnan Li、Yu Lan、Qiuling SongDOI:10.1039/c8cc05390a日期:——Herein, we report an efficient and practical strategy for the synthesis of five types of imidazo[2,1-a]isoquinolines via Cp*RhIII-catalyzed [4+2] annulation of 2-arylimidazoles and α-diazoketoesters, whose structural and substituted diversity at 5- or 6-position can be precisely controlled by the α-diazoketoester coupling partners. Compared with previous reports, in this study, we merged two attractive
-
Mild and Highly Efficient Method for the Synthesis of 2-Arylbenzimidazoles and 2-Arylbenzothiazoles作者:Kiumars Bahrami、M. Mehdi Khodaei、Fardin NaaliDOI:10.1021/jo8010232日期:2008.9.1A new, convenient method for the syntheses of 2-substituted benzimidazole and benzothizole is described. Short reaction times, large-scale synthesis, easy and quick isolation of the products, excellent chemoselectivity, and excellent yields are the main advantages of this procedure.
-
Magnetic nanoparticle-supported DABCO tribromide: a versatile nanocatalyst for the synthesis of quinazolinones and benzimidazoles and protection/deprotection of hydroxyl groups作者:Amin Rostami、Omid Pourshiani、Yahya Navasi、Neda Darvishi、Shaghayegh SaadatiDOI:10.1039/c7nj00479f日期:——supported on magnetic Fe3O4 nanoparticles (MNPs-DABCO tribromide) as a novel heterogeneous tribromide type compound was found to be an efficient and reusable nanocatalyst for the one-pot synthesis of 2-arylquinazolin-4(3H)-ones and 2-aryl-1H-benzo[d]imidazoles through oxidative cyclization of aldehydes with 2-aminobenzamides and 1,2-phenylenediamine, respectively. Also, MNPs-DABCO tribromide catalyzed tr
表征谱图
-
氢谱1HNMR
-
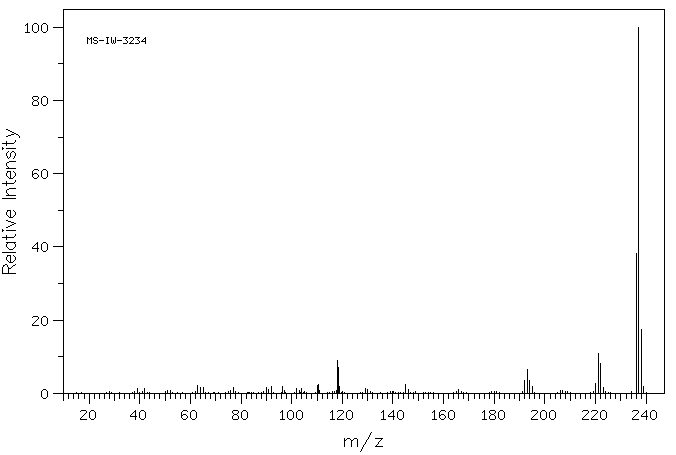
质谱MS
-
碳谱13CNMR
-
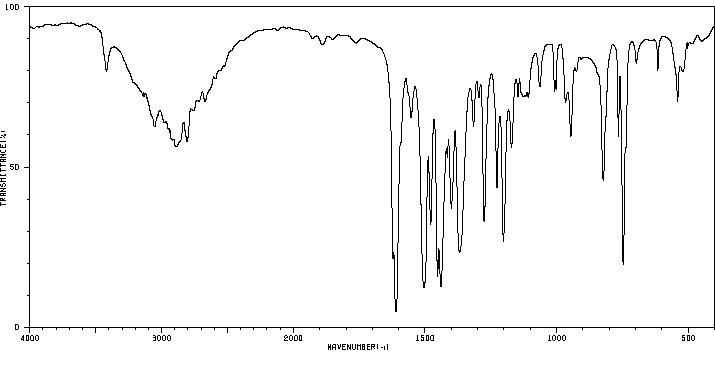
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
麦穗宁
马哌斯汀
颜料橙62
顺式-5,6-二氢-4,5-二甲基-4H-咪唑并[1,5,4-De]喹喔啉
韦罗肟
青菌灵
雷贝拉唑钠
雷贝拉唑硫醚N-氧化物
雷贝拉唑砜 N-氧化物
雷贝拉唑砜
雷贝拉唑杂质2
雷贝拉唑 N-氧化物
雷贝拉唑
阿苯达唑砜
阿苯达唑杂质L
阿苯达唑杂质J(EP)
阿苯达唑杂质J
阿苯达唑杂质F
阿苯达唑杂质14
阿苯达唑杂质13
阿苯达唑亚砜
阿苯达唑
阿苯哒唑砜-D3
阿苯哒唑-D3
阿地本旦
阿司咪唑-d3
阿司咪唑
钠4-[5-氯-2-[(E,3E)-3-[6-氯-1-乙基-3-(4-磺酸丁基)-5-(三氟甲基)苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-6-(三氟甲基)苯并咪唑-1-鎓-1-基]丁烷-1-磺酸盐
邻甲磺酰胺基苯乙酸
那地特罗
达比加群酯杂质M
达比加群酯杂质4
达比加群酯杂质1
达比加群酯杂质
达比加群酯N-氧化物
达比加群酯
达比加群脂杂质10
达比加群甲酯杂质
达比加群杂质J
达比加群杂质J
达比加群杂质F
达比加群杂质E
达比加群杂质D
达比加群杂质C5
达比加群杂质38
达比加群杂质13
达比加群杂质10(DABRC-10)
达比加群杂质10