二氨基马来腈 | 1187-42-4
中文名称
二氨基马来腈
中文别名
二氨基顺丁烯二腈;2,3-二氨基顺丁烯二腈;二氨基马来酰腈;2,3-二氨基-2-丁烯二腈;DAMN
英文名称
diaminomaleonitrile
英文别名
2,3-diaminomaleonitrile;DAMN;(Z)-2,3-diaminobut-2-enedinitrile
CAS
1187-42-4
化学式
C4H4N4
mdl
MFCD00001870
分子量
108.103
InChiKey
DPZSNGJNFHWQDC-ARJAWSKDSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:178-179 °C (lit.)
-
沸点:192.71°C (rough estimate)
-
密度:1,36 g/cm3
-
溶解度:5000毫克/升
-
暴露限值:NIOSH: IDLH 25 mg/m3
-
物理描述:Diaminomaleonitrile is a brown powder. (NTP, 1992)
-
稳定性/保质期:
如果按照规格进行使用和储存,则不会发生分解,目前没有已知的危险反应。请避免接触氧化物、光线以及酸性物质。
计算性质
-
辛醇/水分配系数(LogP):-1
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:99.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S24/25,S36
-
危险类别码:R25
-
WGK Germany:3
-
海关编码:2926909090
-
危险品运输编号:UN 3439 6.1/PG 3
-
危险类别:6.1
-
包装等级:III
-
危险性防范说明:P261,P280,P301+P310,P311
-
危险性描述:H301,H311,H331
-
储存条件:请将贮藏器密封,并将其存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
模块 1. 化学品
1.1 产品标识符
: 二氨基马来腈
产品名称
1.2 鉴别的其他方法
DAMN
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 4)
急性毒性, 吸入 (类别 4)
急性毒性, 经皮 (类别 4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H312 皮肤接触有害。
H332 吸入有害。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 防护服。
事故响应
P301 + P312 如果吞咽并觉不适: 立即呼叫解毒中心或就医。
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P312 如感觉不适,呼救中毒控制中心或医生.
P322 具体处置(见本标签上提供的急救指导)。
P330 漱口。
P363 沾污的衣服清洗后方可再用。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: DAMN
别名
: C4H4N4
分子式
: 108.1 g/mol
分子量
组分 浓度或浓度范围
Diaminomaleonitrile
-
化学文摘登记号(CAS 1187-42-4
No.) 214-697-6
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 深棕
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 178 - 179 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强酸, 强碱, 强氧化剂, 强还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 通过皮肤吸收有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 3439 国际海运危规: 3439 国际空运危规: 3439
14.2 联合国运输名称
欧洲陆运危规: NITRILES, TOXIC, SOLID, N.O.S. (Diaminomaleonitrile)
国际海运危规: NITRILES, TOXIC, SOLID, N.O.S. (Diaminomaleonitrile)
国际空运危规: Nitriles, solid, toxic, n.o.s. (Diaminomaleonitrile)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
简介
含腈的精细化学品因其具有较高的抗代谢稳定性、热稳定性、化学稳定性和膜渗透性等特点,在农药和医药的合成中被广泛应用。其中,二氨基马来腈类化学品是应用最为广泛的含腈精细化学品之一,由于其持效期长、杀虫谱广、低毒性和高药效等特性,这种化合物在新农药的合成以及荧光探针领域得到了越来越广泛的应用,市场前景十分广阔。
合成方法将不同浓度(分别为0%、10%、20% 和 100%)的二苯基二硫醚装入带有磁力搅拌棒的小瓶中。向每个小瓶中依次加入丙酮氰醇(500.0 mg,5.87 mmol,4.0 当量)、三乙胺(164 µL,1.18 mmol,0.8 当量)和少量乙腈,确保反应混合物总体积达到2 mL。在磁力搅拌下将混合物加热至70°C,并持续搅拌4小时后过滤、重结晶以获得二氨基马来腈。
二氨基马来腈用作合成各种杂环化合物的重要起始原料,还能用于制备医药、农药和染料等中间体。此外,它也是聚酯用分散染料的重要原料之一。
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Bedel, C., Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1923, vol. 176, p. 168 - 171摘要:DOI:
-
作为产物:参考文献:名称:Bedel, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1923, vol. 176, p. 168摘要:DOI:
-
作为试剂:描述:methyl 5-[3-,6-dihydro-3-methyl-2,6-dioxo-4-(trifluoromethyl)-1(2H)-pyrimidinyl]-1,2-benzisothiazole-3-glyoxylate 、 邻苯二胺 以 乙醇 、 二氨基马来腈 为溶剂, 生成 3-[3-(3,4-dihydro-3-oxo-2-quinoxalinyl)-1,2-benziso-thiazol-5-yl]-1-methyl-6-(trifluoromethyl)uracil参考文献:名称:Herbicidal 3 -heterocyclic substituted benzisothiazole and benzisoxazole compounds摘要:提供了具有结构式I的3-杂环取代苯并异噻唑和苯并噁唑化合物,其中Q、X、X1和Z的定义如权利要求1中所述。还提供了包含这些化合物的组合物和方法,用于控制不良植物物种。公开号:US20020028748A1
文献信息
-
Biphenylenes-xxxi作者:John W. Barton、Michael C. Goodland、Ken J. Gould、John F.W. Mcomie、W.Roderick Mound、Sadiq A. SalehDOI:10.1016/s0040-4020(01)99488-8日期:1979.1As possible routes to 1,4-diazabiphenylene and its 2,3-disubstituted derivatives we have studied the condensation of benzocyclobutene-1,2-dione (BBD) with various 1,2-diamines. Instead of giving the 1,4-diazabiphenylene ring system, BBD reacted with ethylenediamine, diaminomaleonitrile, 4,5-diaminopyrimidine, 2-aminopyridine, also 2,3- and 3,4-diaminopyridine to give, respectively, 2-o-carboxyphenylimidazolidinium作为制备1,4-二氮杂联苯及其2,3-二取代衍生物的可能途径,我们研究了苯并环丁烯1,2-二酮(BBD)与各种1,2-二胺的缩合反应。BBD与提供1,4-二氮杂联苯环体系不同的是,它与乙二胺,二氨基马来腈,4,5-二氨基嘧啶,2-氨基吡啶,2,3-和3,4-二氨基吡啶反应分别生成2-邻-羧基苯基咪唑啉乙酸甲酯4,3,4-二氰基-2,5-二氢[2,5] benzodiazocine -1,6-二酮10,4-氨基-5A,9B二氢-5-,9B二羟基苯并[3' ,4' ] cyclobuta [1' ,2'-4,5]咪唑并[1,2 ç ]嘧啶14,图5A,图9b二氢5α,9B二羟基苯并[3' ,4 '] cyclobuta [1',2'- 4,5]咪唑[1,中,4-氨基衍生物16,后者的,和两性离子18的4 -氨基-3-(2-羧基亚苄基)。然而,BBD与4,5- diaminobenzotriazole反应,得到预期的1
-
Angiotensin II antagosist 1-biphenylmethylimidazole compounds and their申请人:Sankyo Company, Limited公开号:US05616599A1公开(公告)日:1997-04-01Compounds of the following formula (I) or the formula (I).sub.p : ##STR1## wherein R.sup.1 is alkyl or alkenyl; R.sup.2 and R.sup.3 are hydrogen, alkyl, alkenyl, cycloalkyl, aralkyl, aryl, or aryl fused to cycloalkyl; R.sup.4 is hydrogen, alkyl, alkanoyl, alkenoyl, arylcarbonyl, alkoxycarbonyl, tetrahydropyranyl, tetrahydrothiopyranyl, tetrahydrothienyl, tetrahydrofuryl, a group of formula --SiR.sup.a R.sup.b R.sup.c, in which R.sup.a, R.sup.b and R.sup.c are alkyl or aryl, alkoxymethyl, (alkoxyalkoxy)methyl, haloalkoxymethyl, aralkyl, aryl or alkanoyloxymethoxycarbonyl; R.sup.5 is carboxy or --CONR.sup.8 R.sup.9, wherein R.sup.8 and R.sup.9 hydrogens or alkyl, or R.sup.8 and R.sup.9 together form alkylene; R.sup.6 is hydrogen, alkyl, alkoxy or halogen; R.sup.7 is carboxy or tetrazol-5-yl; R.sub.p.sup.1 is hydrogen, alkyl, cycloalkyl or alkanoyl; R.sub.p.sup.2 is a single bond, alkylene or alkylidene; R.sub.p.sup.3 and R.sub.p.sup.4 are each hydrogen or alkyl; R.sub.p.sup.6 is carboxy or tetrazol-5-yl; and X.sub.p is oxygen or sulfur; and pharmaceutically acceptable salts and esters thereof. The compounds are AII receptor antagonists and thus have hypotensive activity and can be used for the treatment and prophylaxis of hypertension. The compounds may be prepared by reacting a biphenylmethyl compound with an imidazole compound.以下式(I)或式(I).sub.p的化合物:其中R.sup.1是烷基或烯基;R.sup.2和R.sup.3是氢、烷基、烯基、环烷基、芳基烷基、芳基或与环烷基融合的芳基;R.sup.4是氢、烷基、烷酰基、烯酰基、芳基羰基、烷氧羰基、四氢吡喃基、四氢硫代吡喃基、四氢噻吩基、四氢呋喃基,具有式--SiR.sup.a R.sup.b R.sup.c的基团,其中R.sup.a、R.sup.b和R.sup.c是烷基或芳基、烷氧甲基、(烷氧基烷氧基)甲基、卤代烷氧甲基、芳基烷基、芳基或烷酰氧甲氧羰基;R.sup.5是羧基或--CONR.sup.8 R.sup.9,其中R.sup.8和R.sup.9是氢或烷基,或R.sup.8和R.sup.9一起形成亚烷基;R.sup.6是氢、烷基、烷氧基或卤素;R.sup.7是羧基或四唑-5-基;R.sub.p.sup.1是氢、烷基、环烷基或烷酰基;R.sub.p.sup.2是单键、烷基或烷基亚甲基;R.sub.p.sup.3和R.sub.p.sup.4分别是氢或烷基;R.sub.p.sup.6是羧基或四唑-5-基;X.sub.p是氧或硫;以及其药学上可接受的盐和酯。这些化合物是AII受体拮抗剂,因此具有降压活性,可用于治疗和预防高血压。这些化合物可以通过将联苯甲基化合物与咪唑化合物反应制备而成。
-
Efficient Synthesis of Olmesartan Medoxomil, an Antihypertensive Drug作者:Karrothu Srihari Babu、Mallepalli Srinivasa Reddy、Amirisetty Ravindranath Tagore、Gade Srinivas Reddy、Sony Sebastian、Mudunuru Satish Varma、Gandu Venkateswarlu、Apurba Bhattacharya、Padi Pratap Reddy、Ramasamy Vijaya AnandDOI:10.1080/00397910802372558日期:2008.12.31Abstract This document describes a simple and robust process for the synthesis of olmesartan medoxomil. This tailored process allows us to synthesize olmesartan medoxomil on a large scale with 50% overall yield. Also, our process has excellent control of the impurity profile in all the stages.
-
ANTI-VIRAL COMPOUNDS, COMPOSITIONS, AND METHODS OF USE申请人:Leivers Martin Robert公开号:US20090226398A1公开(公告)日:2009-09-10Disclosed are compounds and compositions of Formula (I), pharmaceutically acceptable salts and solvates thereof, and their preparation and uses for treating viral infections mediated at least in part by a virus in the Flaviviridae family of viruses.披露了公式(I)的化合物和组合物、药物可接受的盐和溶剂化物,以及它们的制备和使用,用于治疗至少部分由黄病毒科病毒家族中的病毒介导的病毒感染。
-
Calix[4]arene Schiff bases—potential ligands for fluorescent studies作者:Mary F. Mahon、John McGinley、A. Denise Rooney、John M.D. WalshDOI:10.1016/j.tet.2008.09.091日期:2008.12derivatives incorporating imine substituents were synthesised from the Schiff-base reaction of 25,27-bis[2-[(1-formyl-2-phenyl)oxy]ethyl]-p-tert-butylcalix[4]arene (1) with the appropriate amine or hydrazone. All compounds were characterised by various spectroscopic and analytical techniques, and in three cases, by X-ray crystallographic studies. In the case of compound 2, inclusion compounds were synthesised
表征谱图
-
氢谱1HNMR
-
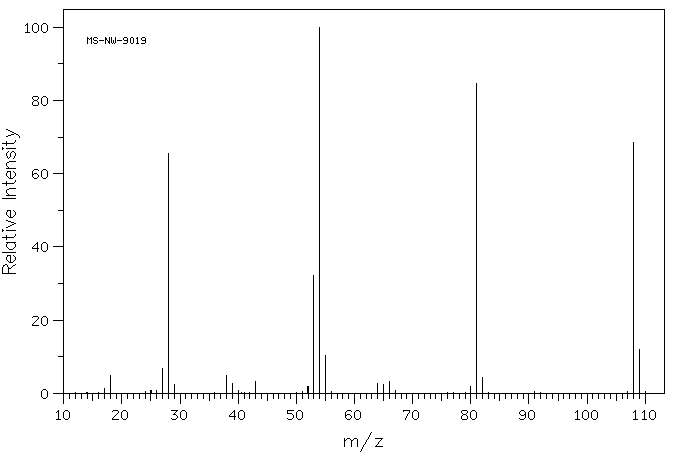
质谱MS
-
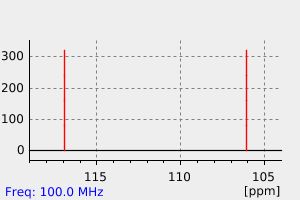
碳谱13CNMR
-
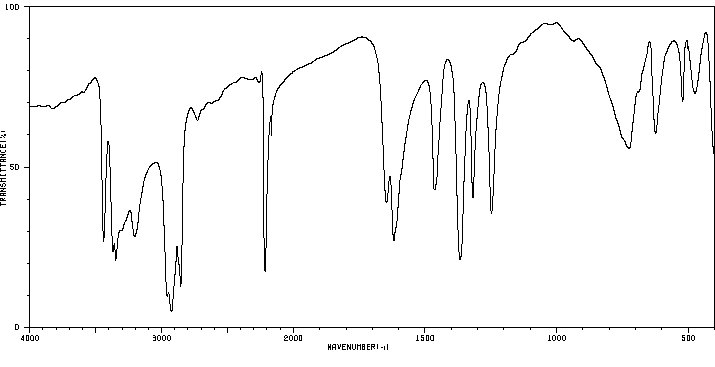
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷