N-(2,4-二硝基苯基)乙酰胺 | 610-53-7
中文名称
N-(2,4-二硝基苯基)乙酰胺
中文别名
2,4-二硝-N-乙醯苯胺;2,4-二硝乙醯胺苯;2,4-二硝基乙酰苯胺
英文名称
2,4-dinitroacetanilide
英文别名
N-(2,4-dinitrophenyl)acetamide;N-(2,4-nitrophenyl)acetamide;N-acetyl-2,4-dinitroaniline;2',4'-dinitroacetanilide;acetic acid-(2,4-dinitro-anilide)
CAS
610-53-7
化学式
C8H7N3O5
mdl
MFCD00024398
分子量
225.161
InChiKey
BRZYMGKDOVQJGX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
本品有毒。
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:16
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:121
-
氢给体数:1
-
氢受体数:5
安全信息
-
海关编码:2924299090
-
储存条件:设备需要密封,确保车间通风良好,并要求操作人员穿戴好劳动保护用品。在运输和储存时,应按照有毒化学品的规定进行处理。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : N-(2,4-Dinitrophenyl)-acetamide
CAS-No. : 610-53-7
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C8 H7 N3 O5 C8H7N3O5
Molecular Weight : 225,16 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Avoid
breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 0,701
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
从乙醇中析出的该物质为针状体结晶,熔点为121°C(范围:125-126°C),能溶于苯、乙醚和乙醇。
用途该产品用作硫化染料中间体。
生产方法生产过程如下:首先将400公斤乙酐加入酰化反应锅中并搅拌。在10至15分钟内逐步加入2,4-二硝基苯胺(97-02-9)共550公斤,然后升温至40°C。接下来,分1小时缓缓加入4.2公斤硫酸,并将温度升至115-120°C,维持恒温6小时。
反应完成后,升温至120-125°C进行真空蒸馏回收乙酸,直至回收量达到150公斤时结束蒸馏。接下来,在木桶中加入1000升水并放入1000公斤碎冰,使水温降至5°C以下,并在半小时内将上述酰化反应物缓慢加入水中。随后,在30°C或更低的温度下过滤产物,滤饼用冷水洗涤至pH值为6-7,在80°C以下条件下干燥得到成品。最终收率为95%。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-硝基乙酰苯胺 o-nitroacetanilide 552-32-9 C8H8N2O3 180.163 —— N'-(2,4-Dinitrophenyl)-N-(4-methylphenyl)ethanimidamide 128915-25-3 C15H14N4O4 314.301 2,4-二硝基苯胺 2,4-Dinitroanilin 97-02-9 C6H5N3O4 183.123 对硝基乙酰苯胺 N-(4-Nitrophenyl)acetamide 104-04-1 C8H8N2O3 180.163 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(2-氨基-4-硝苯基)乙酰胺 4-nitro-2-aminoacetanilide 53987-32-9 C8H9N3O3 195.178 —— N-(2,4-dinitro-phenyl)-diacetamide —— C10H9N3O6 267.198 2,4-二硝基苯胺 2,4-Dinitroanilin 97-02-9 C6H5N3O4 183.123 2,4-二氨基-乙酰苯胺 N1-Acetyl-1,2,4-triamino-benzol 6373-15-5 C8H11N3O 165.195
反应信息
-
作为反应物:描述:参考文献:名称:1-芳基-3-(4-甲氧基苄基)脲作为潜在不可逆的糖原合酶激酶3抑制剂:合成和生物学评估。摘要:糖原合酶激酶3(GSK-3)因其多因素参与阿尔茨海默氏病的发病机理而闻名。在这项研究中,通过将亲电子战斗部结合到环支架上,合成了拟议的Cys199靶向GSK-3β共价抑制剂,合成了1-芳基-3-(4-甲氧基苄基)脲的苯并噻唑和苯并咪唑组。与参考抑制剂AR-A014418(1,IC50 = 0.072)相比,腈取代的苯并咪唑基尿素2b(IC50 = 0.086±0.023 µM)和卤代甲基酮取代的苯并咪唑基尿素9b(IC50 = 0.13±0.060 µM)具有较高的GSK-3β抑制活性。 ±0.043)。结果表明,对2b和9b作为GSK-3β的潜在共价抑制剂的进一步研究,因为靶向相互作用可能提供改善的激酶选择性。DOI:10.1016/j.bmcl.2019.04.049
-
作为产物:描述:N-乙酰苯胺 在 sodium nitrate 、 dipotassium peroxodisulfate 作用下, 以 乙腈 为溶剂, 反应 24.0h, 以51%的产率得到N-(2,4-二硝基苯基)乙酰胺参考文献:名称:无过渡金属的受保护苯胺的单硝化或二硝化摘要:摘要 提出了酰胺辅助芳烃硝化,分别使用NaNO2和NaNO3作为单硝化剂和双硝化剂,可以实现受保护苯胺的单硝化和二硝化。这种发散合成不含过渡金属和酸,具有广泛的底物范围、低成本和邻位选择性。图形概要DOI:10.1080/00397911.2020.1752730
文献信息
-
Reaction of aromatic amides with phenyl iodosylacetate: an oxidative rearrangement作者:K. Swaminathan、N. VenkatasubramanianDOI:10.1039/p29750001161日期:——oxidative rearrangement of primary amides with phenyl iodosylacetate (PIA) yields the corresponding acylamines in acetic acid. The kinetics of the reaction of PIA with a number of substituted benzamides have been studied in solvent acetic acid and also in acetic acid–water mixtures. Electron-releasing substituents in the benzene rIng accelerate the rate of the reaction while electron withdrawing substituents
-
Bismuth Oxide Perchlorate as a Highly Efficient Catalyst for Heteroatom Acylation Under Solvent-Free Conditions作者:Asit K. Chakraborti、Rajesh Gulhane、ShivaniDOI:10.1055/s-2003-41442日期:——Bismuth oxide perchlorate efficiently catalyzes the acetylation of structurally diverse phenols, alcohols, thiols, and amines under solvent free conditions. Sterically hindered and electron deficient phenols are acetylated in excellent yields with stoichiometric amounts of Ac 2 O at room temperature. Acylation of acid-sensitive alcohols is carried out efficiently without competitive side reactions
-
Zirconium(IV) Chloride as a New, Highly Efficient, and Reusable Catalyst for Acetylation of Phenols, Thiols, Amines, and Alcohols under Solvent-Free Conditions作者:Asit K. Chakraborti、Rajesh GulhaneDOI:10.1055/s-2004-815442日期:——chloride has been found to be a new. highly efficient, and reusable catalyst for acetylation of structurally diverse phenols, thiols, amines and alcohols under solvent-free condtions. Acetylation of sterically hindered and electron deficient phenols is achieved in excellent yields with stoichiometric amounts of Ac 2 O at room temperature. Acid-sensitive alcohols undergo acetylation with excellent chemoselectivity
-
Perchloric acid adsorbed on silica gel as a new, highly efficient, and versatile catalyst for acetylation of phenols, thiols, alcohols, and amines
-
Fluoroboric acid adsorbed on silica gel as a new and efficient catalyst for acylation of phenols, thiols, alcohols, and amines作者:Asit K. Chakraborti、Rajesh GulhaneDOI:10.1016/s0040-4039(03)00683-x日期:2003.4Fluoroboric acid supported on silica gel efficiently catalyzes acylation of structurally diverse phenols, alcohols, thiols, and amines under solvent free conditions. Acid-sensitive alcohols are smoothly acylated without competitive side reactions.
表征谱图
-
氢谱1HNMR
-
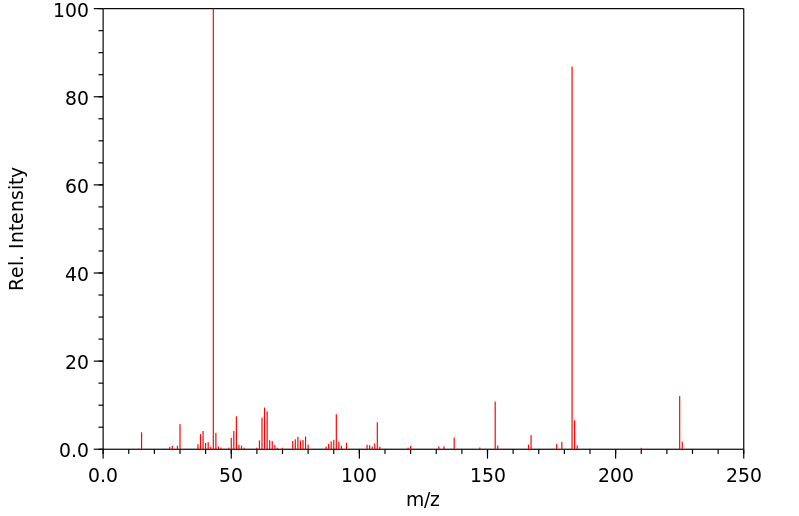
质谱MS
-
碳谱13CNMR
-
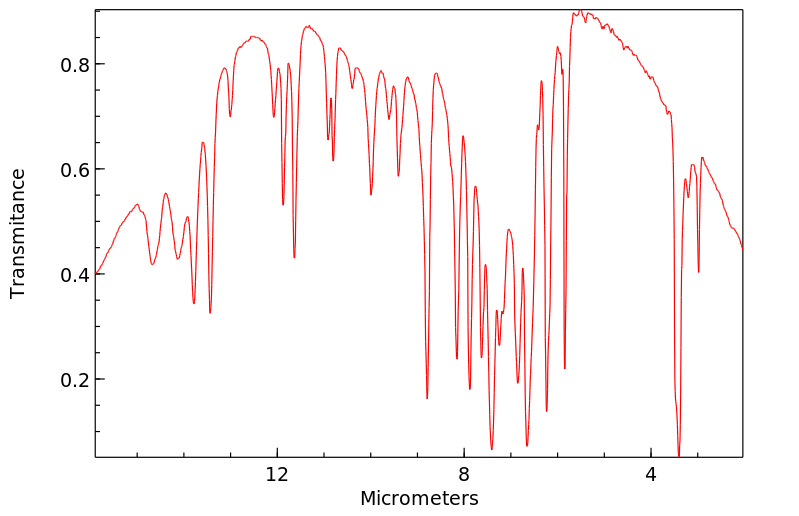
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫