双苯乙甲胺 | 13977-33-8
中文名称
双苯乙甲胺
中文别名
N-二甲苯乙胺;地美维林;N,N-双(2-苯基乙基)-n-甲胺
英文名称
N-methyl-N-phenethyl-2-phenylethan-1-amine
英文别名
demelverine;N,N-Bis-phenaethyl-methylamin;N-methyl-2-phenyl-N-(2-phenylethyl)ethanamine
CAS
13977-33-8
化学式
C17H21N
mdl
——
分子量
239.36
InChiKey
XVWQQNARVMHZBP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160 °C
-
沸点:204 °C(Press: 25 Torr)
-
密度:0.998±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:18
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2921499090
-
储存条件:应存于室温、避光且干燥密封的环境中。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 n-甲基苯基乙胺 N-Methyl-N-phenethylamine 589-08-2 C9H13N 135.209
反应信息
-
作为反应物:参考文献:名称:Granger,R. et al., Chimica Therapeutica, 1968, vol. 3, p. 129 - 135摘要:DOI:
-
作为产物:描述:ethyl N-methyl-N-(phenethoxycarbonyl)glycinate 在 potassium carbonate 作用下, 以 水 、 乙腈 为溶剂, 反应 12.25h, 生成 双苯乙甲胺参考文献:名称:氨基甲酸酯挤出烷基化合成叔胺摘要:碱性胺是许多具有生物活性的天然产物和药物的关键元素。鉴于其固有的反应性,在靶向合成过程中通常需要保护碱性胺,这会导致保护/脱保护序列的浪费。我们报告了一种分步经济的方法,能够在仲胺通过 CO 2的正式挤出转化为叔胺之前将其保护为氨基甲酸酯。该方法适用于 iboga 生物碱 (±)-conodusine A 和 (±)-conodusine B 的合成。DOI:10.1021/acs.orglett.2c02516
文献信息
-
[EN] NEW PROCESS FOR THE SYNTHESIS OF TAPENTADOL AND INTERMEDIATES THEREOF<br/>[FR] NOUVEAU PROCÉDÉ POUR LA SYNTHÈSE DE TAPENTADOL ET SES INTERMÉDIAIRES申请人:ARCHIMICA SRL公开号:WO2012001571A1公开(公告)日:2012-01-05The object of the present invention is a new process for the synthesis of tapentadol, both as free base and in hydrochloride form, which comprises the step of alkylation of the ketone (VII) to yield the compound (VIII), as reported in Diagram 1, with high stereoselectivity due to the presence of the benzyl group as substituent of the amino group. It was surprisingly found that this substitution shifts the keto-enol equilibrium towards the desired enantiomer and amplifies the capacity of the stereocenter present in the compound (VII) to orient the nucleophilic addition of the organometallic compound at the carbonyl towards the desired stereoisomer. This substitution thus allows obtaining a considerable increase of the yields in this step, and consequently allows significantly increasing the overall yield of the entire tapentadol synthesis process. A further object of the present invention is constituted by the tapentadol free base in solid form, obtainable by means of the process of the invention. Still another object of the invention is represented by the crystalline forms I and II of the tapentadol free base. A further object of the present invention is the mixture of the crystalline forms I and II of the tapentadol free base.本发明的对象是一种用于合成替本多尔(tapentadol)的新工艺,包括将酮(VII)烷基化以得到化合物(VIII),如图1所示,其具有高立体选择性,这是由于苄基作为氨基的取代基存在。令人惊讶地发现,这种取代将酮-烯醇平衡转向所需的对映体,并增强了化合物(VII)中存在的立体中心引导有机金属化合物在羰基上的亲核加成朝向所需立体异构体的能力。这种取代因此允许在这一步骤中获得产量的显着增加,从而显著提高整个替本多尔合成过程的总产量。本发明的另一个对象是通过本发明的方法获得的固态替本多尔游离碱。本发明的另一个对象是替本多尔游离碱的结晶形式I和II。本发明的另一个对象是替本多尔游离碱的结晶形式I和II的混合物。
-
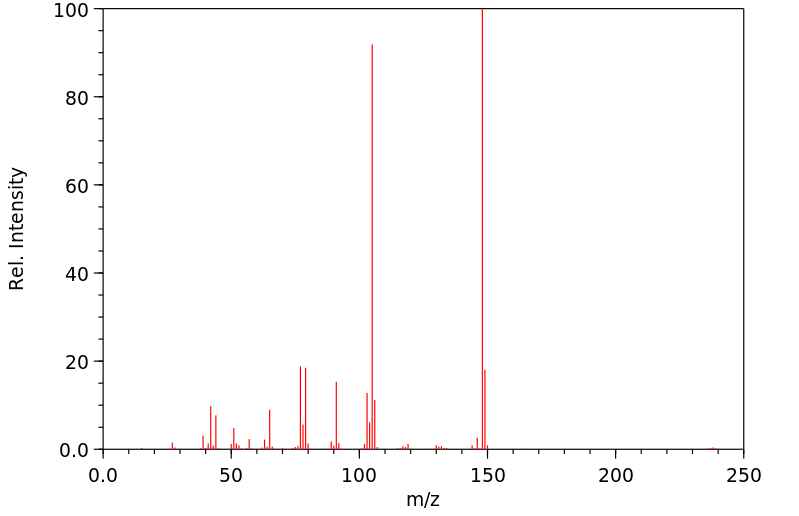
Substituenteneinfluss bei der massenspektrometrischen Fragmentierung: Untersuchungen an N-methyl-?,??-diphenyl-di�thylaminen. 19. Mitteilung �ber das massenspektrometrische Verhalten von Stickstoffverbindungen作者:Peter A. Weibel、Manfred HesseDOI:10.1002/hlca.19730560728日期:1973.11.7Some different substituted N-methyl-β,β′-diphenyl-diethylamines (I) were investigated mass spectrometrically. The main fragmentations and their genesis are summarized in Scheme 2. The molecular ion generates the major fragmentions a (m/e 148) and b (m/e (147 + X)); c (m/e 105) is formed from a and d (m/e (104+X)) from b by further decomposition. The logarithms of the ratios of the relative ion intensities
-
Characterization of Route Specific Impurities Found in Methamphetamine Synthesized by the Leuckart and Reductive Amination Methods作者:Vanitha Kunalan、Niamh Nic Daéid、William J. Kerr、Hilary A. S. Buchanan、Allan R. McPhersonDOI:10.1021/ac9005588日期:2009.9.1Impurity profiling of seized methamphetamine can provide very useful information in criminal investigations and, specifically, on drug trafficking routes, sources of supply, and relationships between seizures. Particularly important is the identification of “route specific” impurities or those which indicate the synthetic method used for manufacture in illicit laboratories. Previous researchers have缉获的甲基苯丙胺杂质分析可为刑事调查提供非常有用的信息,特别是关于贩毒路线、供应来源和缉获之间关系的信息。特别重要的是识别“特定路线”杂质或那些表明在非法实验室制造时使用的合成方法的杂质。以前的研究人员提出了 Leuckart 和还原胺化 (Al/Hg) 制备方法所特有的杂质。然而,迄今为止,重要的是,这两种合成方法尚未在使用内部合成的盐酸甲基苯丙胺的单一研究中进行比较,因此具有已知的合成来源。使用相同的原料,1-苯基-2-丙酮(P2P),通过Leuckart和还原胺化方法合成了40批甲基苯丙胺盐酸盐(每种方法20批)。分别提取碱性和酸性杂质并通过 GC/MS 进行分析。从该对照研究中,报告了 Leuckart 方法的两种路线特定杂质和还原胺化方法的一种路线特定杂质。评估了这些途径特定杂质的批内和批间变化。此外,最近推荐用于甲基苯丙胺分析的“目标杂质”的变化与它们在使用 Leuckart
-
Method for preparing high purity diphenyl carbonate申请人:MITSUBISHI GAS CHEMICAL COMPANY, INC.公开号:EP0722931A1公开(公告)日:1996-07-24A method for preparing a high purity diphenyl carbonate. The diphenyl carbonate is distilled in the presence of a basic substance. The present invention provides high purity diphenyl carbonate using a simple method. A high molecular aromatic polycarbonate containing substantially no polymerization inhibitor and without coloring is easily prepared from the purified diphenyl carbonate.
-
PROCESS FOR THE SYNTHESIS OF TAPENTADOL AND INTERMEDIATES THEREOF申请人:Motta Giuseppe公开号:US20130178644A1公开(公告)日:2013-07-11The object of the present invention is a new process for the synthesis of tapentadol, both as free base and in hydrochloride form, which comprises the step of alkylation of the ketone (VII) to yield the compound (VIII), as reported in Diagram 1, with high stereoselectivity due to the presence of the benzyl group as substituent of the amino group. It was surprisingly found that this substitution shifts the keto-enol equilibrium towards the desired enantiomer and amplifies the capacity of the stereocenter present in the compound (VII) to orient the nucleophilic addition of the organometallic compound at the carbonyl towards the desired stereoisomer. This substitution thus allows obtaining a considerable increase of the yields in this step, and consequently allows significantly increasing the overall yield of the entire tapentadol synthesis process. A further object of the present invention is constituted by the tapentadol free base in solid form, obtainable by means of the process of the invention. Still another object of the invention is represented by the crystalline forms I and II of the tapentadol free base. A further object of the present invention is the mixture of the crystalline forms I and II of the tapentadol free base.本发明的目标是一种新的合成替帕酚(tapentadol)的过程,包括自由基和盐酸盐形式,其中包括烷基化酮(VII)的步骤,得到化合物(VIII),如图1所示,由于苄基作为氨基取代基的存在,具有高立体选择性。令人惊讶的是,这种取代使得酮-烯醇平衡向所需的对映体偏移,并增强了化合物(VII)中立体中心定向有机金属化合物在羰基上的亲核加成的能力,以朝向所需的立体异构体。因此,这种取代允许在此步骤中获得相当大的产量增加,并因此显着提高整个替帕酚合成过程的总产量。本发明的另一个目标是通过本发明的过程获得固体形式的替帕酚自由基。本发明的另一个目标是替帕酚自由基的结晶形式I和II。本发明的另一个目标是替帕酚自由基结晶形式I和II的混合物。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫