喹喔啉-2,3-二甲腈 | 17132-92-2
中文名称
喹喔啉-2,3-二甲腈
中文别名
——
英文名称
quinoxaline-2,3-dicarbonitrile
英文别名
2,3-Quinoxalinedicarbonitrile
CAS
17132-92-2
化学式
C10H4N4
mdl
——
分子量
180.169
InChiKey
MFFPGENYYFVBPT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:210-215 °C
-
沸点:443.0±45.0 °C(Predicted)
-
密度:1.38±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:14
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:73.4
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2933990090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氨基-2-喹噁啉甲腈 3-amino-2-quinoxalinecarbonitrile 36597-16-7 C9H6N4 170.173 2-喹喔啉甲腈4-氧化物 2-Chinoxalin-carbonitril-4-oxid 53688-02-1 C9H5N3O 171.158 2-氯喹喔啉-3-甲腈 2-chloroquinoxaline-3-carbonitrile 40254-89-5 C9H4ClN3 189.604
反应信息
-
作为反应物:描述:参考文献:名称:取代的四-2,3-吡嗪并四氮杂卟啉。第二部分。双(三正己基甲硅烷氧基)硅衍生物摘要:通过2,3-二氰基吡嗪、2,3-二氰基-5,6-二苯基吡嗪、2,3-二氰基喹喔啉、2,3-二氰基-苯并[f]的缩合获得取代的四-2,3-吡嗪并四氮杂卟啉的二氯硅配合物在尿素、喹啉和三正丁胺存在下,喹喔啉和 2,3-二氰基-二苯并[f,h]喹喔啉与四氯化硅。Si-Cl 键在浓 H2SO4 中水解,然后用 0.01 N NaOH 和 NH3 水溶液处理,得到相应的二氢氧化物,通过与三(正己基)反应转化为双(三正己基甲硅烷氧基)硅衍生物) 在三正丁胺存在下的 3-甲基吡啶(2,4,6-可力丁)中的硅烷。中心硅原子上的轴向三正己基甲硅烷氧基取代基可防止有机溶剂中的聚集,允许详细研究结构修饰对四氮杂酞菁电子光谱的影响。我们的数据显示,每个苯并环加成角缩合到四-2,3-喹喔啉基四氮杂卟啉上,会诱导...DOI:10.1139/v96-189
-
作为产物:参考文献:名称:3-氨基-2-喹喔啉甲腈。具有潜在细胞毒性活性的新型融合喹喔啉摘要:从3-氨基-2-喹喔啉腈1,4-二氧化物1开始,通过化学修饰2-氰基和3-氨基制备了一系列新的喹喔啉衍生物。硝化3-氨基-2-喹喔啉腈3,得到7-硝基衍生物6。3的重氮化得到3-氯化合物9。从9获得2,3-喹喔啉二甲腈14。吡啶并[4,5- b ]喹喔啉15和16是通过将14与水合肼缩合而制备的。三唑并[4,5- b ]喹喔啉18,鉴定出异噻唑并[4,5- b ]喹喔啉20和两个吡唑并[3,4- b ]喹喔啉21和22。测试了化合物在有氧和低氧细胞中的细胞毒性作用。DOI:10.1002/jhet.5570310506
文献信息
-
Facile Synthesis of Hydrazine Derivatives of 5H-Pyrrolo[3,4-b]pyrazine and 1H-Pyrrolo[3,4-b]quinoxaline作者:Olga Hordiyenko、Igor Rudenko、Irina Zamkova、Oleksandr Denisenko、Angelina Biitseva、Axelle Arrault、Andrei TolmachevDOI:10.1055/s-0033-1340042日期:——convenient and efficient route for the chemoselective synthesis of carbohydrazide, arenesulfonylhydrazide, and thiosemicarbazone derivatives of 5H-pyrrolo[3,4-b]pyrazine and 1H-pyrrolo[3,4-b]quinoxaline from the corresponding dinitriles and substituted hydrazines was elaborated. The synthesis of starting pyrazine-2,3-dicarbonitrile was sufficiently improved by using concentrated HCl as a catalyst. A convenient
-
Quinoxalines. XXVII. The cyanation of 2-substituted quinoxaline 4-oxides with trimethylsilyl cyanide.作者:Chihoko IIJIMA、Akira MIYASHITADOI:10.1248/cpb.38.661日期:——The deoxy-cyanation of 2-substituted quinoxaline 4-oxides (Ia-k) with trimethylsilyl cyanide in the presence of 1, 8-diazabicyclo[5.4.0]undec-7-ene gave the corresponding 3-substituted 2-quinoxalinecarbonitriles (IIa-k). However, in the case of 2-(p-tolylsulfonyl)quinoxaline 4-oxide (II), the substitution with cyanide ion proceeded together with deoxy-cyanation to give 2, 3-quinoxalinedicarbonitrile (III).
-
Heterocyclic Colorants Based on Diazabenzoisoindoles申请人:Heckmann Heino公开号:US20070264600A1公开(公告)日:2007-11-15The invention is directed to novel colorants of formula (I), wherein A represents a group of general formulas (II), (III), (IV) or (V), and B represents a substituted or unsubstituted ortho-C6-C18 arylene, wherein C and D represent an alicyclic or heteroyclic group.该发明涉及公式(I)的新型着色剂,其中A代表一般公式(II)、(III)、(IV)或(V)的一个基团,B代表取代或未取代的邻位C6-C18芳基,其中C和D代表脂环或杂环基团。
-
A Green One-Pot Synthesis of vic-Amidino (Hetero)aromatic Acids from 1,2-Dinitriles作者:Olga Hordiyenko、Volodymyr Tkachuk、Iryna OmelchenkoDOI:10.1055/s-0036-1588933日期:——Phthalonitrile undergoes partial hydration in MeOH–H2O media in the presence of an equimolar amount of NaOH to afford 2-carbamimidoylbenzoic acid in good yield in one step. This and similar vic-amidino (hetero)aromatic acids also could be synthesized from corresponding 1,2-dinitriles by hydrolysis in aqueous MeOH catalyzed by an equimolar amount of NaOH of in situ generated 1,1-dimethoxy-1H-isoindol-3-amine
-
The first porphyrin–subphthalocyaninatoboron(<scp>iii</scp>)-fused hybrid with unique conformation and intramolecular charge transfer behavior作者:Yuehong Zhang、Juwon Oh、Kang Wang、Dongju Shin、Xiaopeng Zhan、Yingting Zheng、Dongho Kim、Jianzhuang JiangDOI:10.1039/c6cc05383a日期:——Porphyrin and subphthalocyaninatoboron(III) chromophores have been fused through quinoxaline moiety, resulting in the first porphyrin-subphthalocyaninatoboron(III)-fused hybrid with intramolecular charge transfer from tetrapyrrole/tripyrrole chromophores to the quinoxaline moiety. The unique plane-bowl...
表征谱图
-
氢谱1HNMR
-
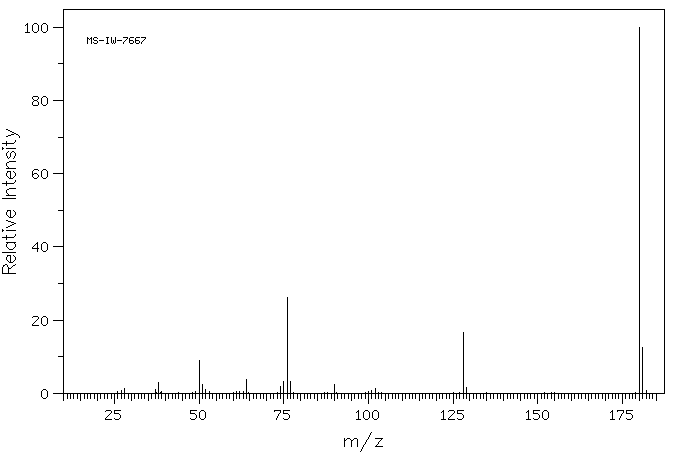
质谱MS
-
碳谱13CNMR
-
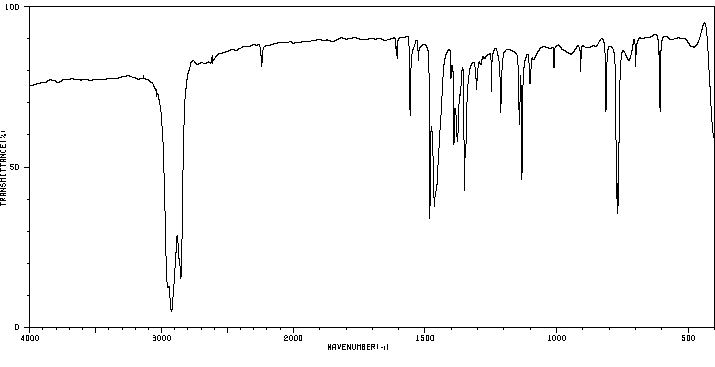
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮