2-硝基噻吩 | 609-40-5
中文名称
2-硝基噻吩
中文别名
2-硝噻吩
英文名称
2-nitrothiophene
英文别名
——
CAS
609-40-5
化学式
C4H3NO2S
mdl
MFCD00005425
分子量
129.139
InChiKey
JIZRGGUCOQKGQD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:43-45 °C (lit.)
-
沸点:224-225 °C (lit.)
-
密度:1,364 g/cm3
-
闪点:201 °F
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:1.55
-
稳定性/保质期:
一、基本性质:能随水蒸气挥发。
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:74.1
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:T
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R68
-
WGK Germany:3
-
海关编码:2934999090
-
危险品运输编号:OTH
-
RTECS号:XN0030000
-
危险性防范说明:P501,P261,P270,P202,P201,P271,P264,P280,P308+P313,P362+P364,P301+P312+P330,P302+P352+P312,P304+P340+P312,P405
-
危险性描述:H302+H312+H332,H341
-
储存条件:贮存: 将密器密封后,储存在密封的主要容器中,并放置在阴凉、干燥的地方。
SDS
| Name: | 2-Nitrothiophene tech. 85% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 609-40-5 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 609-40-5 | 2-Nitrothiophene | 85 | 210-190-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Light sensitive.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Store protected from light.
Storage:
Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances. Dark room.
Store protected from light.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 609-40-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: yellow to beige-brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 224 - 225 deg C @ 760.00mmHg
Freezing/Melting Point: 43.00 - 45.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C4H3NO2S
Molecular Weight: 129.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures. May discolor on exposure to light.
Conditions to Avoid:
Incompatible materials, light, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents, strong bases.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 609-40-5: XN0030000 LD50/LC50:
Not available.
Carcinogenicity:
2-Nitrothiophene - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 609-40-5: No information available.
Canada
CAS# 609-40-5 is listed on Canada's NDSL List.
CAS# 609-40-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 609-40-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-碘-5-硝基噻吩 2-iodo-5-nitrothiophene 6277-18-5 C4H2INO2S 255.036 2-溴-5-硝基噻吩 2-bromo-5-nitrothiophene 13195-50-1 C4H2BrNO2S 208.035 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,5-dinitrothiophene 59434-05-8 C4H2N2O4S 174.137 2-碘-5-硝基噻吩 2-iodo-5-nitrothiophene 6277-18-5 C4H2INO2S 255.036 5-硝基-2-甲基噻吩 2-methyl-5-nitrothiophene 42297-94-9 C5H5NO2S 143.166 2-溴-5-硝基噻吩 2-bromo-5-nitrothiophene 13195-50-1 C4H2BrNO2S 208.035 2-硝基噻吩-3-胺 3-amino-2-nitrothiophene 52003-20-0 C4H4N2O2S 144.154 —— 2-nitro-thiophen-3-ol 128496-62-8 C4H3NO3S 145.139 2-硝基-3-甲基噻吩 3-methyl-2-nitrothiophene 32059-75-9 C5H5NO2S 143.166
反应信息
-
作为反应物:描述:参考文献:名称:Stadler, Chemische Berichte, 1885, vol. 18, p. 1491,2316摘要:DOI:
-
作为产物:参考文献:名称:Nitration of Organolithiums and Grignards with Dinitrogen Tetroxide: Success at Melting Interfaces摘要:DOI:10.1021/ja963658e
-
作为试剂:描述:参考文献:名称:Steinkopf; Luetzkendorf, Justus Liebigs Annalen der Chemie, 1914, vol. 403, p. 48摘要:DOI:
文献信息
-
Palladium-catalyzed four-component carbonylative synthesis of 2,3-disubstituted quinazolin-4(3H)-ones: Convenient methaqualone preparation作者:Jin-Bao Peng、Hui-Qing Geng、Wei Wang、Xinxin Qi、Jun Ying、Xiao-Feng WuDOI:10.1016/j.jcat.2018.06.007日期:2018.9A palladium-catalyzed four-component carbonylative cyclization reaction for the synthesis of 2,3-disubstituted quinazolin-4(3H)-ones has been developed. A range of different 2,3-disubstituted quinazolin-4(3H)-one derivatives were prepared in moderate to good yields employing simple and readily accessible 2-iodoanilines, nitro compounds and acid anhydrides as the synthetic precursors. Mo(CO)6 acted
-
Pd-Catalyzed Silicon Hydride Reductions of Aromatic and Aliphatic Nitro Groups作者:Ronald J. Rahaim、Robert E. MaleczkaDOI:10.1021/ol052120n日期:2005.10.1[reaction: see text] Room-temperature reduction of aromatic nitro groups to amines can be accomplished in high yield, with wide functional group tolerance and short reaction times (30 min) using a combination of palladium(II) acetate, aqueous potassium fluoride, and polymethylhydrosiloxane (PMHS). Replacing PMHS/KF with triethylsilane allows aliphatic nitro groups to be reduced to their hydroxylamines.
-
Vicarious nucleophilic substitution of hydrogen in nitroderivatives of five-membered heteroaromatic compounds作者:Mieczyslaw Makosza、Ewa KwastDOI:10.1016/0040-4020(95)00445-e日期:1995.7Nitro derivatives of thiophene, furan and pyrrole react with carbanions containing leaving groups giving products of replacement of hydrogen with functionalized alkyl substituents. Many specific features of this reaction are discussed.
-
Ultrasonically Assisted Rate Enhancements in Trichloroisocyanuric Acid/DMF/NaNO<sub>2</sub>Triggered Nitration of Aromatic Compounds and Decarboxylative Nitration of<font>α</font>,<font>β</font>-Unsaturated Acids作者:Mukka Satish Kumar、Kamatala Chinna Rajanna、Marri Venkateswarlu、Purugula Venkanna、Pondichery Kuppuswamy SaiprakashDOI:10.1080/00397911.2015.1075044日期:2015.10.2Abstract An efficient and safe method for nitration of aromatic compounds and decarboxylative nitration of α,β-unsaturated acids was developed using trichloroisocyanuric acid (TCICA)/dimethylformamide (DMF) in the presence of NaNO2. The reaction times of conventional protocol reduced from 8–10 h to 1.0–1.5 h (60–90 min) under sonication, even though the yields are comparable under both the conditions
-
Novel approach to synthesis of substituted 3-aminoquinolines from nitroarenes and protected ethyl aminocrotonate作者:Robert Bujok、Andrzej Kwast、Piotr Cmoch、Zbigniew WróbelDOI:10.1016/j.tet.2009.11.060日期:2010.1The addition of mono- and dianions of ethyl N-pivaloyl-3-aminocrotonate to substituted nitroarenes, followed by action of silylating or acylating agent, leads to 3-aminoquinoline carboxylic acid derivatives. Hydrolysis and decarboxylation of the latter, carried out efficiently under relatively mild conditions, afford 3-aminoquinolines diversely substituted in the benzo-fused ring.
表征谱图
-
氢谱1HNMR
-
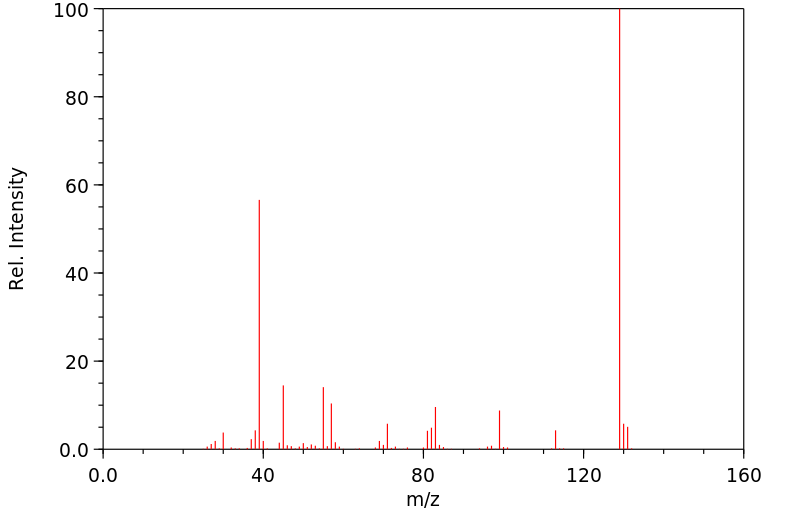
质谱MS
-
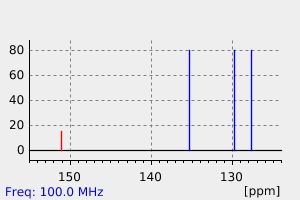
碳谱13CNMR
-
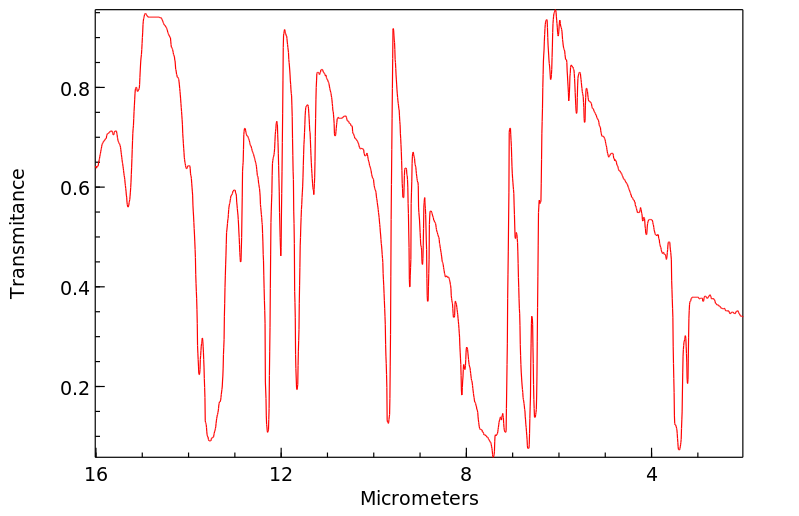
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯