3,3'-二氯氧化偶氮苯 | 139-24-2
中文名称
3,3'-二氯氧化偶氮苯
中文别名
——
英文名称
3,3'-dichloroazoxybenzene
英文别名
1,2-bis(3-chlorophenyl)diazene oxide;3,3’-dichloroazoxybenzene;3,3′-dichloroazoxybenzene;3,3'-di-chloro-azoxybenzene;m,m'-Dichloroazoxybenzene;3,3'-azoxychlorobenzene;(3-chlorophenyl)-(3-chlorophenyl)imino-oxidoazanium
CAS
139-24-2;71297-98-8
化学式
C12H8Cl2N2O
mdl
——
分子量
267.114
InChiKey
SXCDGVDBGGCCLV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:41.1
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,3'-二氯偶氮苯 3,3'-dichloroazobenzene 15426-14-9 C12H8Cl2N2 251.115
反应信息
-
作为反应物:参考文献:名称:偶氮苯的热反应摘要:偶氮苯 (1) 在 245–250 °C 下加热,得到偶氮苯 (2)、2-羟基偶氮苯 (3)、2-羟基偶氮苯 (4)、4-羟基偶氮苯 (5) 和 4-羟基偶氮苯 (6)。化合物4和6主要由α-异构体组成。在偶氮甲苯的反应中,它们的甲基被氧化成甲酰基。有人提出4和6是1到3和5热重排的中间体。DOI:10.1246/bcsj.49.754
-
作为产物:参考文献:名称:在锌介导的将芳族硝基还原为oxy氧基化合物中作为氧气的调节剂摘要:描述了一种简单有效的方案,可通过在[bmim] [BF 4 ]和水的混合物中使用锌和NH 4 Cl将硝基芳烃还原为相应的乙氧基衍生物。将硝基选择性还原为a氧基归因于氧对锌金属的迄今未知的调节作用。DOI:10.1016/j.tetlet.2009.02.122
文献信息
-
Synthesis of Azoxybenzenes by Reductive Dimerization of Nitrosobenzene作者:Yu-Feng Chen、Jing Chen、Li-Jen Lin、Gary Jing ChuangDOI:10.1021/acs.joc.7b01887日期:2017.11.3Herein we report an effective and simple preparation method of substituted azoxybenzenes by reductive dimerization of nitrosobenzenes. This procedure requires no additional catalyst/reagent and can be applied to substrates with a wide range of substitution patterns.在本文中,我们报道了通过亚硝基苯的还原二聚作用制备取代的乙氧基苯的有效而简单的方法。该程序不需要额外的催化剂/试剂,并且可以应用于具有广泛取代模式的底物。
-
Controllable synthesis of azoxybenzenes and anilines with alcohol as the reducing agent promoted by KOH作者:Rui Ping Wei、Feng ShiDOI:10.1080/00397911.2019.1566472日期:2019.3.4Abstract Nitrobenzene and its derivatives can be selectively reduced to the corresponding azoxybenzene and aniline compounds with alcohols as the hydrogen source and KOH as the promoter only by simple changes of reaction conditions. Graphical Abstract
-
REDUCTION OF AROMATIC AND ALIPHATIC NITRO COMPOUNDS BY SODIUM HYDROGEN TELLURIDE作者:Atsuhiro Osuka、Hirohito Shimizu、Hitomi SuzukiDOI:10.1246/cl.1983.1373日期:1983.9.5Various nitro compounds were effectively reduced by sodium hydrogen telluride in good yields. Thus, reductive conversion of unhindered nitrobenzenes to azoxybenzenes, sterically hindered nitrobenzenes to anilines, nitroalkanes to dimer of nitrosoalkanes, and vicinal-dinitroalkane to olefin was achieved.
-
SO2F2-mediated oxidation of primary and tertiary amines with 30% aqueous H2O2 solution作者:Xudong Liao、Yi Zhou、Chengmei Ai、Cuijiao Ye、Guanghui Chen、Zhaohua Yan、Sen LinDOI:10.1016/j.tetlet.2021.153457日期:2021.11highly efficient and selective oxidation of primary and tertiary amines employing SO2F2/H2O2/base system was described. Anilines were converted to the corresponding azoxybenzenes, while primary benzylamines were transformed into nitriles and secondary benzylamines were rearranged to amides. For tertiary amine substrates quinolines, isoquinolines and pyridines, their oxidation products were the corresponding
-
Switchable Selectivity during Oxidation of Anilines in a Ball Mill作者:Rico Thorwirth、Franziska Bernhardt、Achim Stolle、Bernd Ondruschka、Jila AsghariDOI:10.1002/chem.201001702日期:2010.11.22for the direct oxidation of anilines to the corresponding azo and azoxy homocoupling products by using a planetary ball mill was developed. Various oxidants and grinding auxiliaries were tested and a variety of substituted anilines were investigated. It was possible to form chemoselectively either azo, azoxy, or the nitro compounds from reaction of aromatic anilines. The selectivity of the solvent‐free
表征谱图
-
氢谱1HNMR
-
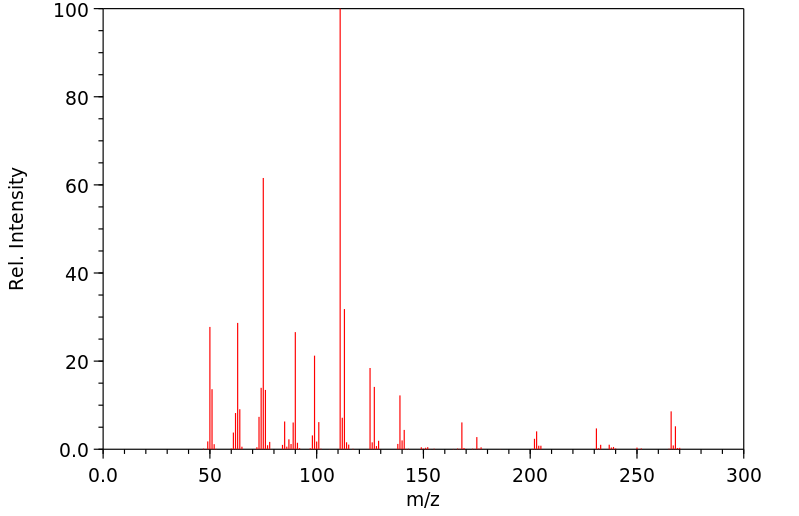
质谱MS
-
碳谱13CNMR
-
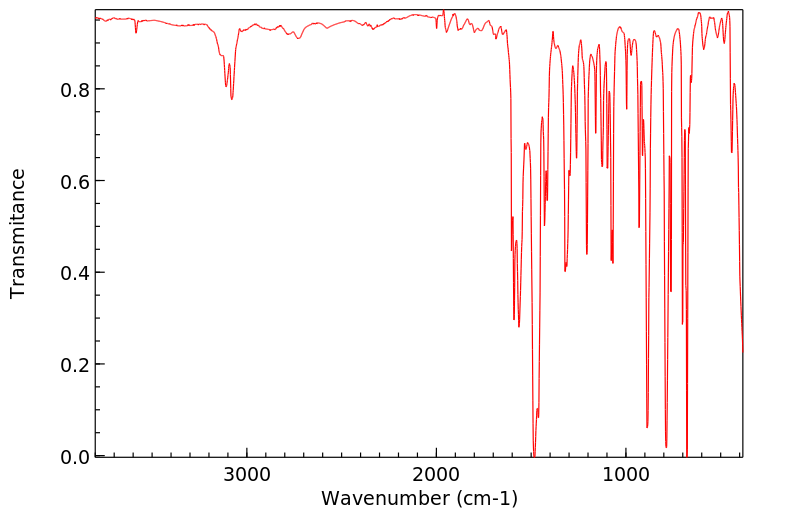
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫