二氧化氯 | 10049-04-4
中文名称
二氧化氯
中文别名
——
英文名称
chlorine dioxide
英文别名
——
CAS
10049-04-4
化学式
ClO2
mdl
——
分子量
67.4518
InChiKey
OSVXSBDYLRYLIG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-59°C
-
沸点:11°C
-
密度:3.09g/L
-
溶解度:微溶于H2O
-
暴露限值:TLV-TWA 0.1 ppm (0.3 mg/m3); (ACGIH, MSHA, OSHA, and NIOSH); TLV-STEL 0.3 ppm (ACGIH); IDLH 10 ppm (NIOSH).
-
介电常数:1.7(77.0℃)
-
LogP:-3.22--2.9 at 20℃
-
物理描述:Chlorine dioxide hydrate, frozen is an orange colored solid, appearing as a block of ice, with a faint odor of chlorine. It may only be shipped in the frozen state and then only by private or contract motor carrier. The melting point of the hydrate is around 30°F. If it should thaw and further warm up, chlorine dioxide gas is given off. The gas is toxic by inhalation. The gas and liquid are violently decomposed by organic materials. The gas will decompose explosively at temperatures below the boiling point of water. It is used to bleach wood pulp, fats and oils; in processing flour, and for water purification. Chlorine dioxide is a yellow to reddish gas or a red-brown liquid below 52 deg. F. with an unpleasant odor similar to chlorine.
-
颜色/状态:Yellowish-brown gas
-
气味:... Unpleasant odor similar to chlorine and nitric acid.
-
闪点:NA (Gas) ? (Liquid)
-
蒸汽密度:2.3 (Air = 1)
-
蒸汽压力:101 kPa at 20 °C /758 mm Hg at 20 °C/
-
稳定性/保质期:
-
分解:When heated to decomposition it emits toxic fumes of /chloride/.
-
燃烧热:Standard net heat of combustion (gas): -102.5 kJ/mol
-
汽化热:30 kJ/mol at 11 °C
-
电离电位:10.36 eV
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:3
-
可旋转键数:0
-
环数:None
-
sp3杂化的碳原子比例:None
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
ADMET
代谢
二氧化氯和亚氯酸盐主要通过代谢转化为氯离子。在大鼠单次口服(灌胃)给予放射性标记的二氧化氯后72小时内,尿液中共收集到的放射性物质中,大约87%是氯离子,而在血浆样本中,氯离子占80%。亚氯酸盐是另一个主要代谢物,分别占尿液和血浆样本中放射性物质的约11%和21%。氯酸盐是尿液中放射性物质的一个小组成部分。类似地,在大鼠口服给予放射性标记的亚氯酸盐后的72小时尿液中,大约85%的放射性物质是氯离子;其余以亚氯酸盐的形式存在。
Both chlorine dioxide and chlorite are primarily metabolized to chloride ion. At 72 hours following single oral (gavage) administration of radiolabeled chlorine dioxide in rats, chloride ion accounted for approximately 87% of the radioactivity that had been collected in the urine and 80% of the radioactivity in a plasma sample. Chlorite was the other major metabolite, accounting for approximately 11 and 21% of the radioactivity in the urine and plasma samples, respectively. Chlorate was a minor component of the radioactivity in the urine. Similarly, chloride ion accounted for approximately 85% of the radioactivity in the 72-hour urine collection of rats that had been orally administered radiolabeled chlorite; the remainder in the form of chlorite.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:二氧化氯是一种淡黄色的气体,有类似于氯气和硝酸的难闻气味。其用途包括漂白纤维素、纸浆、面粉、皮革、脂肪和油、纺织品和蜂蜡。它还用于水的净化、水的口感和异味的控制、清洁和脱脂皮革、制造氯化盐,以及作为氧化剂、杀菌剂、消毒剂和除臭剂。二氧化氯在非常低的浓度下,最少接触时间为30到60秒,对各种测试的微生物都非常有效。人体研究:过度暴露的潜在症状包括眼睛、鼻子和喉咙的刺激,以及咳嗽、喘息、支气管炎和肺水肿。在空气中接触低浓度气体的工人偶尔会出现眼睛刺激和看到光晕,但这些影响与呼吸道的刺激相比是轻微的。支气管镜检查和活检显示,12名工人中有7人患有轻度慢性支气管炎。只有两名在检查前刚刚接触过二氧化氯的工人出现了呼吸系统影响的物理症状。急性暴露于二氧化氯的工人发展出了反应性气道功能障碍综合征(RADS),一种职业性哮喘,以及一种被称为反应性上呼吸道功能障碍综合征(RUDS)的上呼吸道反应性疾病。母亲在怀孕期间接触二氧化氯处理的水与新生儿早产之间发现了正相关。母亲饮用经二氧化氯或次氯酸盐消毒的水的儿童出生时,小头围、身长短和新生儿黄疸的发病率较高。二氧化氯在人类白细胞中显示出较弱的遗传毒性。动物研究:二氧化氯通过大鼠吸入非常有毒。毒性症状包括呼吸急促。宏观上,所有接触二氧化氯的动物组都出现了肺水肿和肺气肿,其发生率随着剂量的增加而增加。当通过口服途径给予大鼠二氧化氯溶液时,它是有毒的。接受80毫克二氧化氯/千克体重的两名男性和两名女性死亡,另外两名男性在40毫克/千克体重下也在给药后48小时内死亡。在20毫克/千克体重下没有死亡。在大鼠的发育研究中,没有显示出对生殖功能的损害,并且在接触高达10毫克/千克体重/天的二氧化氯水溶液的大鼠中没有发现发育影响的迹象。在用二氧化氯处理的鼠类中,没有发现微核形成增加,结构或数量染色体畸变也没有增加。然而,二氧化氯增加了用水遗传毒性的检测,包括大肠杆菌和酿酒酵母的检测。在中国仓鼠卵巢细胞中,2.5-15微克/毫升的浓度下,出现了显著的剂量相关性和统计学上显著增加的有染色体畸变的细胞中期数。在代谢激活的存在下,75微克/毫升的浓度下观察到细胞毒性和缺少有丝分裂细胞。在50微克/毫升的浓度下,有染色体畸变的细胞中期的数量增加。生态毒性研究:在海胆接触到250毫克/升二氧化氯浓度时,出现了发育异常。与对照组相比,孵化前畸形增加了6%,发育迟缓增加了2%,孵化后畸形增加了20%,骨骼畸形增加了21%,肠道畸形增加了11%。二氧化氯对海藻鱼苗的存活没有显著影响。在胖头鱼(Pimephales promelas)中,二氧化氯暴露产生了剂量依赖性的鳃病理变化,包括上皮细胞脱落、肥大、增生、板层融合和坏死。即使在出现严重肥大和板层融合的鱼中,4天内也实现了完全恢复。在酸性的pH下,0.1到0.5毫克/升的二氧化氯浓度诱导了蚕豆形成微核,而1-2毫克/升的二氧化氯在中性pH下给出了阳性反应。二氧化氯在TRAdescantia微核试验中产生了阳性反应。
IDENTIFICATION AND USE: Chlorine dioxide is a yellowish-brown gas with unpleasant odor similar to chlorine and nitric acid. Its uses include bleaching cellulose, paper-pulp, flour, leather, fats and oils, textiles, and beeswax. It is also used for purification of water, taste and odor control of water, cleaning and detanning leather, in the manufacture of chloride salts, and as an oxidizing agent, bactericide, antiseptic and deodorizer. Chlorine dioxide was found to be highly effective against various microorganisms tested at very low concentrations in a minimum contact time of 30 to 60 seconds. HUMAN STUDIES: Potential symptoms of overexposure are irritation of eyes, nose, and throat, as well as coughing, wheezing, bronchitis, and pulmonary edema. Workers industrially exposed to low concentrations of the gas in air have been noted occasionally to suffer from irritation of the eyes and to see haloes about lights, but these effects have been minor compared to respiratory irritation. Bronchoscopy and biopsy revealed slight chronic bronchitis in 7 of 12 workers. Only two workers who had been exposed just prior to examination showed physical signs of respiratory effects. Workers acutely exposed to chlorine dioxide developed both reactive airways dysfunction syndrome (RADS), a form of occupational asthma, and an upper airways reactive disorder that was called reactive upper airways disfunction syndrome (RUDS). A positive association was found between exposure of the mother to chlorine dioxide-treated water during pregnancy and prematurity of the newborn. Children born of mothers who drank disinfected water treated with either chlorine dioxide or hypochlorite were noted to have an increased incidence of small cranial circumference, small body length, and neonatal jaundice. Chlorine dioxide was found to be weakly genotoxic in human leukocytes. ANIMAL STUDIES: Chlorine dioxide is very toxic by inhalation in rats. Clinical signs of toxicity included respiratory distress. Macroscopically, pulmonary edema and emphysema were seen in all groups of chlorine dioxide-exposed animals, with the incidence increasing in a dose-related manner. Chlorine dioxide is toxic when administered in solution by the oral route to rats. Two males and two females receiving 80 mg chlorine dioxide/kg body weight died, and a further two males at 40 mg/kg body weight also died within 48 hr of administration. There were no deaths at 20 mg/kg body weight. A developmental study in rats did not demonstrate any impairment of reproductive function, and there were no signs of developmental effects among rats receiving up to 10 mg aqueous chlorine dioxide/kg body weight per day. A negative result was obtained for micronucleus formation, and there were no increases in the number of structural or numerical chromosome aberrations in mice treated with chlorine dioxide. However, chlorine dioxide increased water genotoxicity in assays with E. coli and with S. cerevisiae. In Chinese hamster ovary cells at 2.5-15 ug/mL, there was a marked dose-related, statistically significant increase in the number of metaphases with chromosome aberrations. In the presence of metabolic activation, cell toxicity and an absence of mitotic cells were observed at 75 ug/mL. An increase in the number of metaphases with chromosome aberrations was noted at 50 ug/mL. ECOTOXICITY STUDIES: Developmental abnormalities in the sea urchin were evident at exposure to the chlorine dioxide concentration 250 mg/L. Compared with the control, pre-hatch malformations were 6% higher; retarded development, 2%; post-hatch malformations, 20%; skeletal malformations, 21%; and gut malformations, 11%. Survival of larval kelp bass was not significantly affected by chlorine dioxide. In Fathead minnows (Pimephales promelas) chlorine dioxide exposure produced dose-dependent gill pathology including epithelial lifting, hypertrophy, hyperplasia, lamellar fusion, and necrosis. Complete recovery, even in fish with severe hypertrophy and lamellar fusion, was achieved within 4 days. Chlorine dioxide concentrations ranging from 0.1 to 0.5 mg/L induced micronuclei in Vicia faba at acid pH, while 1-2 mg/L chlorine dioxide gave positive responses at neutral pH. Chlorine dioxide produced positive responses in the Tradescantia micronucleus test.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
分类:D;无法归类为人类致癌性。分类依据:没有找到评估氯二氧化物长期致癌潜能的满意的人类或动物研究。人类致癌性数据:无。动物致癌性数据:无。
CLASSIFICATION: D; not classifiable as to human carcinogenicity. BASIS FOR CLASSIFICATION: No satisfactory human or animal studies assessing the chronic carcinogenic potential of chlorine dioxide have been located. HUMAN CARCINOGENICITY DATA: None. ANIMAL CARCINOGENICITY DATA: None.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
这种物质可以通过吸入被身体吸收。
The substance can be absorbed into the body by inhalation.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,吞食(液体),皮肤和/或眼睛接触
inhalation, ingestion (liquid), skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
眼睛、鼻子、喉咙刺激;咳嗽、喘息、支气管炎、肺水肿;慢性支气管炎
irritation eyes, nose, throat; cough, wheezing, bronchitis, pulmonary edema; chronic bronchitis
来源:The National Institute for Occupational Safety and Health (NIOSH)
吸收、分配和排泄
研究了番茄或哈密瓜熏蒸后(36)Cl-ClO2气体的分布和化学命运,以及影响氯氧酸根副产物形成的主要因素。在大约5毫克的(36)Cl- 中暴露2小时后,大约有22%的生成的(36)Cl- 存在于熏蒸过的番茄上。用水冲洗可以去除14%的放射性氯,而番茄匀浆中含有大约63%的放射性;24%的放射性氯存在于番茄茎疤痕区域。番茄匀浆中的放射性主要由(36)Cl-氯化物(>/= 80%)、(36)Cl-氯酸盐(5至19%)和过氯酸盐(0.5至1.4%)组成。在哈密瓜中,用100毫克(36)Cl- 熏蒸2小时后,生成的(36)Cl- 中有55%存在于哈密瓜上。可食用的哈密瓜果肉中没有检测到放射性残留物(LOQ = 0.3至0.4微克/克);与哈密瓜相关的放射性超过99.9%位于不可食用的外皮上,与种子床相关的放射性小于0.1%。外皮中的放射性以(36)Cl-氯化物(大约86%)、氯酸盐(大约13%)和过氯酸盐(大约0.6%)的形式存在。番茄和哈密瓜中没有(36)Cl-亚氯酸盐残留物。后续研究表明,通过保护熏蒸室免受光源的影响,可以完全消除氯酸盐和过氯酸盐的形成。
The distribution and chemical fate of (36)Cl-ClO2 gas subsequent to fumigation of tomatoes or cantaloupe was investigated as were major factors that affect the formation of chloroxyanion byproducts. Approximately 22% of the generated (36)Cl-ClO2 was present on fumigated tomatoes after a 2 hr exposure to approximately 5 mg of (36)Cl-ClO2. A water rinse removed 14% of the radiochlorine while tomato homogenate contained approximately 63% of the tomato radioactivity; 24% of the radiochlorine was present in the tomato stem scar area. Radioactivity in tomato homogenate consisted of (36)Cl-chloride (>/= 80%), (36)Cl-chlorate (5 to 19%), and perchlorate (0.5 to 1.4%). In cantaloupe, 55% of the generated (36)Cl-ClO2 was present on melons fumigated with 100 mg of (36)Cl-ClO2 for a 2 hr period. Edible cantaloupe flesh contained no detectable radioactive residue (LOQ = 0.3 to 0.4 ug/g); >99.9% of radioactivity associated with cantaloupe was on the inedible rind, with <0.1% associated with the seed bed. Rind radioactivity was present as (36)Cl-chloride (approximately 86%), chlorate (approximately 13%), and perchlorate (approximately 0.6%). Absent from tomatoes and cantaloupe were (36)Cl-chlorite residues. Follow-up studies have shown that chlorate and perchlorate formation can be completely eliminated by protecting fumigation chambers from light sources.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
口服Alcide(次氯酸钠和乳酸)后,在大鼠体内,血浆中的峰值浓度在8小时获得。在144小时时,放射性在血浆中最高,其次是肺、肾、皮肤、骨髓、胃、卵巢、十二指肠、回肠、脾、脂肪、脑、肝和尸体。亚细胞分布显示,肝脏匀浆中的85%活性存在于细胞质中。血浆中总活性的70%位于三氯乙酸上清液中,30%与沉淀蛋白部分结合。尿液排泄是(36)氯消除的主要途径。
After oral administration of Alcide (sodium chlorite and lactic acid) in rats, the peak plasma level was obtained in 8 hr. At 144 hr, radioactivity was highest in plasma followed by lung, kidney, skin, bone marrow, stomach, ovary, duodenum, ileum, spleen, fat, brain, liver, and carcass. Subcellular distribution revealed that 85% of the activity in the liver homogenate resided in the cytosol. 70% of total activity in plasma was located in the trichloroacetic acid supernatant, with 30% bound to the precipitated protein fraction. Urinary excretion accounted for most of the (36)chlorine eliminated.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
一项研究表明,来自水合氯 dioxide 的“氯”(化学形态未特征化)通过口服途径被吸收,具有广泛的分布和快速而广泛的消除。在这项研究中,四组大鼠单次口服灌胃大约 1.5 或 4.5 毫克 36ClO2/千克体重。在给药后长达 48 小时内收集血液样本,并在 72 小时时处死动物,取肾脏、肺、小肠、肝脏、脾脏、胸腺、骨髓和睾丸的样本。除睾丸、皮肤和剩余尸体外,所有组织中均发现 36Cl,尽管这些组织中每个组织的水平均低于给药剂量的 1%。
One study shows that "chlorine" (chemical form not characterized) derived from aqueous chlorine dioxide is absorbed by the oral route, with a wide distribution and rapid and extensive elimination. In this study, groups of four rats received a single oral gavage dose of approximately 1.5 or 4.5 mg 36ClO2/kg body weight. Blood samples were collected for up to 48 hr post-administration, and at 72 hr, animals were killed, with samples taken from kidneys, lungs, small intestine, liver, spleen, thymus, bone marrow, and testes. 36Cl was found in all tissues except testes, skin, and the remaining carcass, although levels in these tissues each accounted for less than 1% of the administered dose.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
大约40%的36Cl单次剂量通过尿液、呼出气体和粪便排出,其中尿液占了大多数(约30%)。
About 40% of /a single dose of/ 36Cl was recovered in urine, expired air, and feces, although the urine accounted for most (about 30%).
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:C
-
职业暴露限值:TWA: 0.1 ppm (0.3 mg/m3), STEL: 0.3 ppm (0.9 mg/m3)
-
立即威胁生命和健康浓度:5 ppm
-
危险品标志:O,T+,N
-
安全说明:S23,S26,S28,S36/37/39,S38,S45,S61
-
危险类别码:R6
-
危险品运输编号:UN 9191
-
储存条件:库房应保持低温、通风和干燥,并注意防火、防高温以及轻搬轻放。需与氧化剂及食品原料分开存放。 二氧化氯气体在受热或光照下容易分解并可能引发爆炸,通常不宜长途运输。若用户(如造纸厂或自来水厂等)确有需求,则建议自产自用。
制备方法与用途
根据提供的信息,二氧化氯的生产方法主要有以下几种:
-
ClO₂发生器法:使用发烟硫酸和还原剂(如甲醇)作为原料。在70±4℃、13.32kPa减压条件下反应,生成二氧化氯。
- 反应式为:
4NaC1O3 + 4H2SO4 + CH3OH → 4 + 4NaHSO4 + HCOOH + 3
- 反应式为:
-
R2法:将氯酸钠与氯化钠混合水溶液(摩尔比为1:1.05)加入反应器,再加入98%硫酸,在35~55℃下反应。生成的二氧化氯和氯气通过空气稀释后进入吸收塔。
- 反应式为:
NaClO3 + NaCl + H2SO4 → <a href=https://www.molaid.com/MS_64647 target="_blank">ClO2</a> + 0.5C12 + Na2SO4 + <a href=https://www.molaid.com/MS_37598 target="_blank">H2O</a>
- 反应式为:
-
R3法:与R2法相似,但使用独特的二氧化氯发生器在低压下操作,可获得更高浓度的二氧化氯溶液(8g/L)。
- 反应式为:
NaClO3 + NaCl + H2SO4 → + 0.5C12 + Na2SO4 +
- 反应式为:
-
R8法:与R3法相似,但使用甲醇作为还原剂,减少了芒硝的副产物。
- 反应式为:
4NaClO3 + 4H2SO4 + CH3OH → 4 + 4NaHSO4 + HCOOH + 3
- 反应式为:
-
R1法(未在文本中详细列出,但可以参考上述方法):使用ClO₂发生器和普通常压反应过程来制备二氧化氯。
每种方法都有其独特的特点和技术要求。例如,R8法则通过使用甲醇作为还原剂减少了副产物的生成。而这些方法都需要注意安全操作,因为二氧化氯是一种高毒性和强氧化性的物质,在生产过程中必须严格控制温度和压力等条件,防止发生爆炸或泄漏。
此外,从职业卫生角度来看,二氧化氯被认为是有较高毒性的化学品,因此在使用时需要遵守相应的安全标准(如TWA 0.28毫克/立方米;STEL 0.84毫克/立方米),并采取适当的安全防护措施。
反应信息
-
作为反应物:描述:参考文献:名称:Stanbury, David M.; Martinez, Roland; Tseng, Ewen, Inorganic Chemistry, 1988, vol. 27, # 23, p. 4277 - 4280摘要:DOI:
-
作为产物:描述:参考文献:名称:溶液中二氧化氯的振动模式特异性光化学反应动力学摘要:我们通过飞秒泵-探针光谱研究了 OClO 在环己烷、乙腈和水中的反应动力学。在所有溶剂中,我们在 403 nm 单色泵探针实验中观察到一个量子拍,时间分辨率为 55 fs,它以 1.3-1.5 ps 的时间常数衰减。由此我们得出结论,与之前的报道相比,并非所有 OClO 分子在用 403 nm 光激发后都会解离。在环己烷和水中,我们在 403 nm 实验中观察到受激发射在 0.5 到 2 ps 之间增加,这似乎与量子拍衰减有关。我们将这些结果解释为 OClO 弯曲模式的振动松弛的结果。从 (ν1,1,0) 到 (ν1,0,0) 的松弛导致具有两倍高跃迁偶极矩的状态人口,这解释了受激发射的增加。进一步证明并非所有 OClO 分子在激发后立即解离,这在确定 femt 中的受激发射贡献中发现...DOI:10.1063/1.1357202
-
作为试剂:描述:参考文献:名称:亚氯酸盐对苯乙烯的环氧反应动力学及其机理:二氧化氯的作用摘要:苯乙烯和对-二甲苯环氧化动力学及其机理的研究描述了在25°C下5-6的pH范围内由亚氯酸盐取代的苯乙烯。在水和水/乙腈中提议的机理包括氯的七个氧化态(-I,0,I,II,III,IV和V),以说明观察到的动力学和产物分布。该模型为氯物种和有机底物混合物中发生的复杂反应提供了异常详细的定量机制,尤其是在使用强氧化剂亚氯酸盐时。通过向反应混合物中加入二氧化氯来实现反应的动力学控制,从而消除了单独使用亚氯酸盐时观察到的大量诱导期。该环氧化剂被确定为二氧化氯,它是由亚氯酸盐与次氯酸反应连续形成的,次氯酸是由环氧化反应产生的ClO产生的。总体化学计量是两个竞争性链式反应的结果,其中反应性中间体ClO与二氧化氯或亚氯酸根离子反应,分别生成次氯酸和氯酸根或氯离子。在高亚氯酸根离子浓度下,HOCl与亚氯酸根反应可快速消除,从而最大程度地减少了HOCl与Cl之间的副反应2.以起始原料。动力学模型可准确预测环氧化合物的选择性(在最佳条件下>DOI:10.1021/ic500512e
文献信息
-
Photoreduction of Pt(IV) Halo-Hydroxo Complexes: Possible Hypohalous Acid Elimination作者:Lasantha A. Wickramasinghe、Paul R. SharpDOI:10.1021/ic402358s日期:2014.2.3detected in photolyzed benzene solutions. Photolysis of 3 or 6 in the presence of 2,3-dimethyl-2-butene (TME) yields the chlorohydrin (2-chloro-2,3-dimethyl-3-butanol), 3-chloro-2,3-dimethyl-1-butene, and acetone, all expected products from HOCl trapping, but additional oxidation products are also observed. Photolysis of mixed chloro-bromo complex 7 with TME yields the bromohydrin (2-bromo-2,3-dimethyl-3-butanol)将浓缩的过氧化氢加至反式Pt(PEt 3)2 Cl(R)[ 1(R = 9-菲基),2(R = 4-三氟甲基苯基)]可以生成反式Pt(PEt 3)2( Cl)(OOH)(OH)(R)[ 5(R = 9-菲基),4(R = 4-三氟甲基苯基)],其中氢过氧配体反式为R。络合物5不稳定并与溶剂CH 2反应CL 2,得到的反式,顺式-Pt(PET 3)2(Cl)的2(OH)(9-菲基)(3)。用HCl处理4可获得类似的反式-顺式-Pt(PEt 3)2(Cl)2(OH)(4-三氟甲基苯基)(6)和HBr产生反式-Pt(PEt 3)2(Br)(Cl)( OH)(4-三氟甲基苯基)(7),其中Br和4-三氟甲基苯基配体是反式的。3或6在313或380 nm处发生光解会导致反式Pt(PEt 3)2 Cl(R)(1或2)。未检测到预期的副产物HOCl,但显示出真实的HOCl溶液在反应条件下会分解。在光解苯溶液中检测到氯苯和其他可将PPh
-
An infrared study of the UV photolysis of chlorine nitrate trapped in various matrices at 11 K作者:Armelle De Saxce、Louise SchriverDOI:10.1016/0009-2614(92)85016-4日期:1992.11Photolysis of matrix isolated chlorine nitrate in argon matrix assisted by reactive matrices (solid nitrogen and oxygen) at 11 K have been carried out by visible and ultraviolet light in the 800—250 nm range. The product identification and relative measurements of the species time evolution were made by Fourier transform infrared spectrometry. Below 300 nm, two main dissociation channels are evidenced
-
Outer-sphere electron-transfer reactions involving the chlorite/chlorine dioxide couple. Activation barriers for bent triatomic species作者:David M. Stanbury、Lynn A. LednickyDOI:10.1021/ja00322a018日期:1984.5Cinetique des reactions redox mettant en jeu le couple ClO 2 |ClO 2 − en solution aqueuse. ClO 2 est reduit par [Co(terpy) 2 ] 2+ pour produire ClO 2 − . ClO 2 − est oxyde par IrCl 6 2− pour produire ClO 2 et IrCl 6 3−Cinetique des reaction 氧化还原金属 en jeu le Couple ClO 2 |ClO 2 − en solution aqueuse。ClO 2 est reduit par [Co(terpy) 2 ] 2+ pour produire ClO 2 − 。ClO 2 − est oxyde par IrCl 6 2− 倒入产品 ClO 2 et IrCl 6 3−
-
Radiation mechanisms. Part 9. A comparative electron spin resonance study of radiation effects in thallous and thallic salts作者:Martyn C. R. Symons、Donald N. ZimmermanDOI:10.1039/dt9760000180日期:——Exposure of thallous salts to high-energy radiation usually yields TlII centres together with paramagnetic centres derived from the anions which show strong charge-transfer interaction with neighbouring thallous ions. Previous work on thallous acetate and nitrate is extended to the perchlorate, and to the corresponding TlIII salts. The latter all gave TlII but centres derived from the anions generally
-
DUAL BIOCIDE GENERATOR申请人:Ecolab USA Inc.公开号:US20160029639A1公开(公告)日:2016-02-04Methods and apparatus for generation of dual biocides are provided. The electrolytic generation of chlorine as a biocide is employed for further generation of additional biocides within a single system or generator, including bromine, iodine, chlorine dioxide, fluorine, or chloramines from their respective salts and/or precursors. A single on-site generating system produces a combination of biocides for applications of use providing cost, safety and efficacy improvements. Methods of using the disinfecting biocides provide a synergistic effect through simultaneous or sequential applications.
表征谱图
-
氢谱1HNMR
-
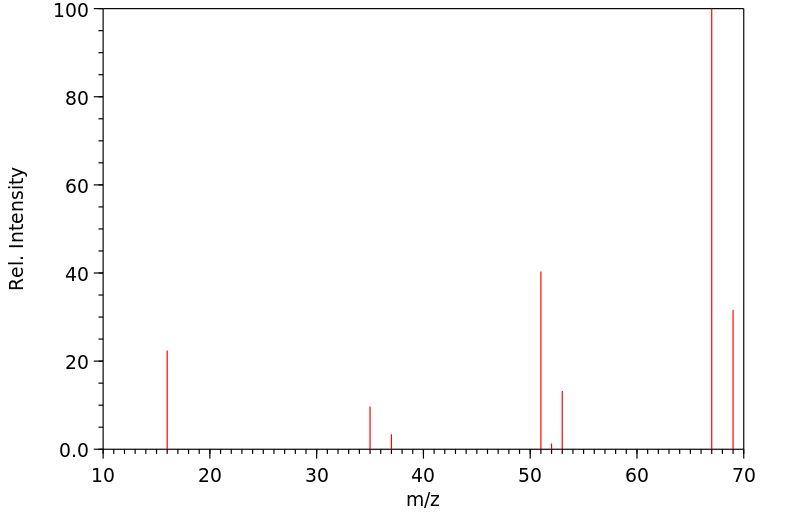
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息