代谢
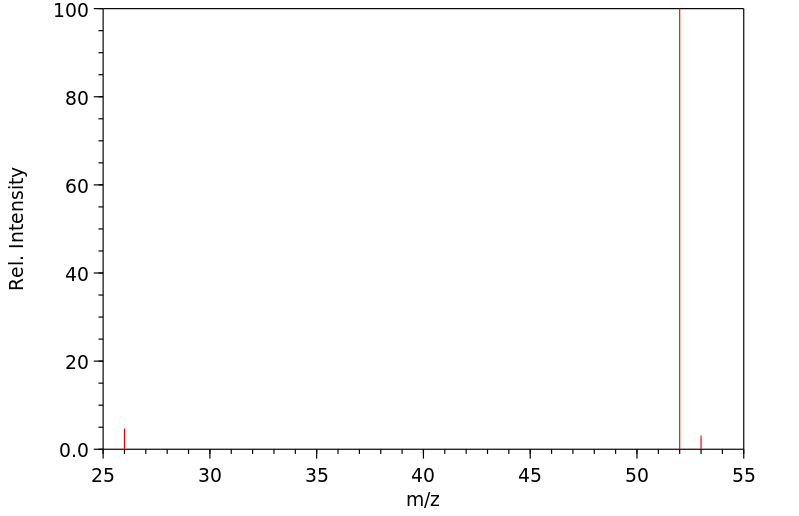
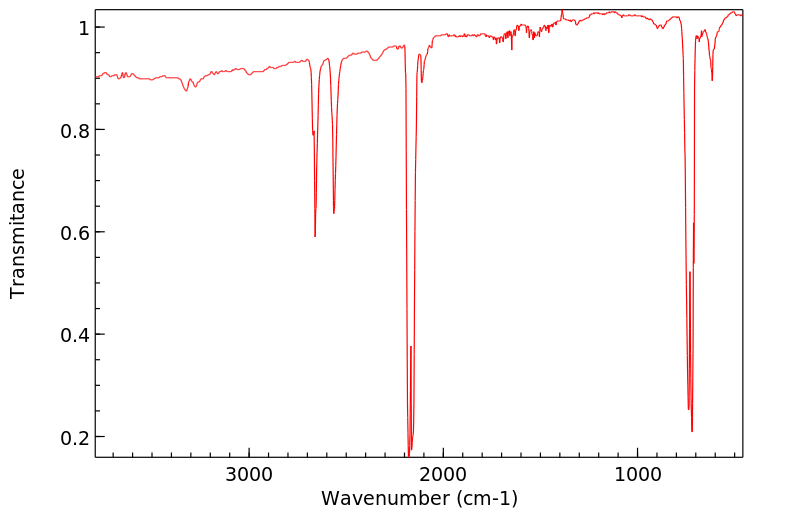
氰化物的暴露可能通过吸入氰化氢气体发生,氰化氢气体是氰化物的一种二聚体。然而,氰化氢在水溶液中会分解成氰离子(CN-)和OCN-离子。分解速率取决于pH值,在碱性介质中(例如,血液中的氢氰酸以H+和CN-的形式处于pH 7.38 - 7.44的平衡状态)比在酸性介质中(例如,胃内容物在pH 3时只有氢氰酸这一种物质)更快。有关氰化氢暴露后在身体组织或体液中形成的氰离子量的报告已经发布;但是,氰离子的量会随着身体组织或体液类型的不同而变化。因此,氰化氢暴露后很难估计身体组织中的氰化物水平。
Exposure to cyanide can ... occur by inhalation of cyanogen gas, a dimer of cyanide. However, cyanogen breaks down in aqueous solution into cyanide ion (CN-1) and OCN- ions. The rate of the breakdown depends on pH and is faster in basic media (e.g., hydrogen cyanide is in equilibrium as H+ and CN- in blood with a pH of 7.38 - 7.44) than in acidic media (e.g., hydrogen cyanide is the only species in stomach contents at a pH of 3). The amount of cyanide ion formed within a body tissue or fluid as a result of exposure to cyanogen has been reported; however, the amount varies with type of body tissue and fluid. Thus, it is difficult to estimate cyanide levels in body tissues after cyanogen exposure.
来源:Hazardous Substances Data Bank (HSDB)