N,N,N,'N'-四乙基对苯二甲酰胺 | 15394-30-6
中文名称
N,N,N,'N'-四乙基对苯二甲酰胺
中文别名
——
英文名称
N1,N1,N4,N4-tetraethylterephthalamide
英文别名
N,N-diethyl terephthalamide;N,N,N',N'-Tetraethylterephthalamide;1-N,1-N,4-N,4-N-tetraethylbenzene-1,4-dicarboxamide
CAS
15394-30-6
化学式
C16H24N2O2
mdl
MFCD00043653
分子量
276.379
InChiKey
GBSCSRXSQKTCPD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:127 °C
-
沸点:419.28°C (rough estimate)
-
密度:1.0209 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:20
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:40.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-propionyl-benzoic acid diethylamide —— C14H19NO2 233.31
反应信息
-
作为反应物:参考文献:名称:777.抗结核化合物。部分X.衍生自季铵化合物的一些反应NN二取代的硫代酰胺摘要:DOI:10.1039/jr9520004067
-
作为产物:参考文献:名称:B 12-铑-钛氧化物杂化催化剂催化可见光驱动的光催化二重反应。摘要:合成了由B 12配合物和铑离子(Rh 3+)改性的氧化钛组成的杂化催化剂,用于可见光驱动的B 12激发的催化反应。杂化催化剂在氧化钛表面上含有4.93×10 -6 molg -1的B 12络合物和5.43×10 -5 molg -1的Rh(III)离子。在作为牺牲试剂的三乙胺(Et 3 N)存在下,杂化催化剂的可见光照射(λ≥420 nm)显示出在B 12的Co(I)状态下典型的390 nm吸收。通过漫反射紫外可见光谱分析监测该配合物,这意味着电子从二氧化钛转移到B 12配合物的Co(III)中心是通过可见光辐射发生的。在室温下,在空气中,由杂化催化剂催化的可见光辐射将三氯化苯转化为N,N-二乙基苯甲酰胺。催化剂的导带电子和价带孔均用于反应以形成酰胺产物。提出了二重反应的反应机理。DOI:10.1016/j.jorganchem.2019.121058
文献信息
-
One-Pot Synthesis of Tertiary Amides from Organic Trichlorides through Oxygen Atom Incorporation from Air by Convergent Paired Electrolysis作者:Zhongli Luo、Kenji Imamura、Yoshihito Shiota、Kazunari Yoshizawa、Yoshio Hisaeda、Hisashi ShimakoshiDOI:10.1021/acs.joc.1c00161日期:2021.4.16A convergent paired electrolysis catalyzed by a B12 complex for the one-pot synthesis of a tertiary amide from organic trichlorides (R-CCl3) has been developed. Various readily available organic trichlorides, such as benzotrichloride and its derivatives, chloroform, dichlorodiphenyltrichloroethane (DDT), trichloro-2,2,2-trifluoroethane (CFC-113a), and trichloroacetonitrile (CNCCl3), were converted
-
Pd/C-Catalyzed Aminocarbonylation of Aryl Iodides via Oxidative C–N Bond Activation of Tertiary Amines to Tertiary Amides作者:Rajendra S. Mane、Bhalchandra M. BhanageDOI:10.1021/acs.joc.5b02385日期:2016.2.5This work reports oxidative N-dealkylation/carbonylation of tertiary amines to tertiary amides by using molecular oxygen as a sole oxidant using a Pd/C catalyst. This protocol is free from ligands, additives, bases, and cocatalysts. Different tertiary amines as well as aryl iodides have been examined for this transformation, providing desired products in good to excellent yield.
-
The ‘inverse electron-demand’ Diels–Alder reaction in polymer synthesis. Part 5: Preparation and model reactions of some electron-rich bis-dienamines作者:András Kotschy、János Faragó、Antal Csámpai、David M SmithDOI:10.1016/j.tet.2004.02.035日期:2004.4The m- and p-phenylene-bridged bis-azolopyridinium salts have been synthesized and converted into the corresponding bis-dienamines by reaction with pyrrolidine. These dienamines react readily with dimethyl 1,2,4,5-tetrazine-dicarboxylate to yield the bis-azolylvinyl-pyridazines.的米-和p -亚苯基桥连的双azolopyridinium盐已经合成,并通过与吡咯烷反应转化成相应的二dienamines。这些二烯胺容易与1,2,4,5-四嗪-二羧酸二甲酯反应,生成双-偶氮基乙烯基-哒嗪。
-
Dilithiated synthons of tertiary benzamides, phthalamides, and 0,0′-aryl dicarbamates作者:R.J. Mills、R.F. Horvath、M.P. Sibi、V. SnieckusDOI:10.1016/s0040-4039(00)98418-1日期:1985.1Dilithiated species 1, 2, and 3, generated by metal-halogen exchange and directed ortho metalation, undergo reaction with electrophiles to afford, in high yield, polysubstituted aromatics 6, 7, and 8 respectively; 9 is formed via a bis anionic Fries rearrangement of 3.
-
Silica-Supported 2,4,6-Trichloro-1,3,5-triazine as an Efficient Reagent for Direct Conversion of Carboxylic Acids to Amides Under Solvent-Free Conditions作者:Ali Khalafi-Nezhad、Abdolkarim Zare、Abolfath Parhami、Mohammad Navid Soltani Rad、Gholam Reza NejabatDOI:10.1080/10426500601047214日期:2007.4.1A very simple and efficient solvent-free method for the direct conversion of carboxylic acids to primary, secondary, tertiary alkyl, and aromatic amides in the presence of the corresponding ammonium salts, silica-supported 2,4,6-trichloro-1,3,5-triazine, and triethylamine is described. The reactions proceed rapidly at room temperature, and the products are obtained in moderate to excellent yields.
表征谱图
-
氢谱1HNMR
-
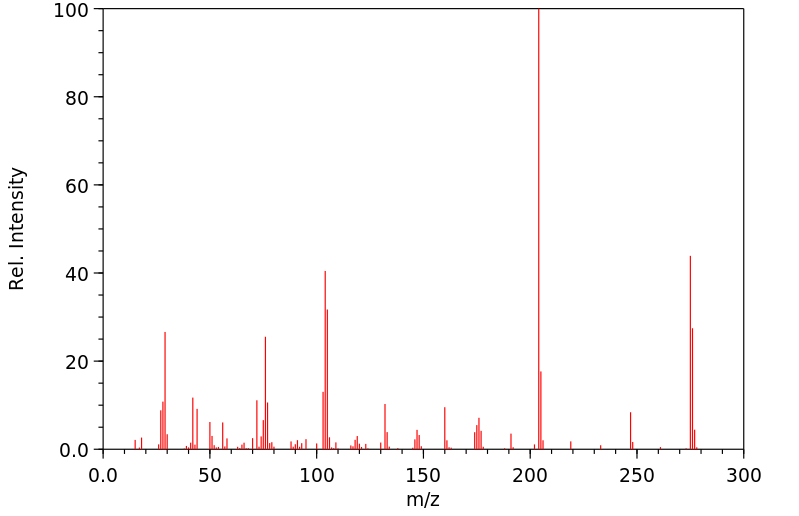
质谱MS
-
碳谱13CNMR
-
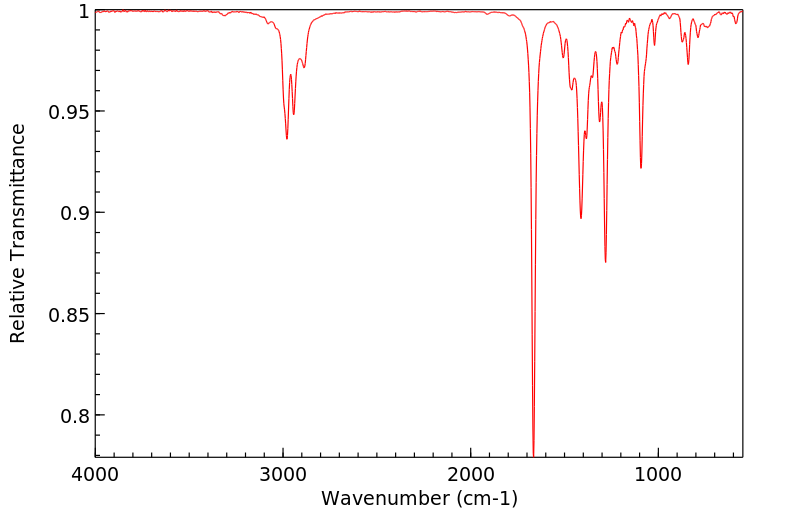
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫