2-甲基-硒氮茚 | 2818-88-4
中文名称
2-甲基-硒氮茚
中文别名
2-甲基苯并硒唑
英文名称
2-methylbenzoselenazole
英文别名
2-Methylbenzoselenazol;2-Methyl-benzselenazol;2-methyl-1,3-benzoselenazole;2-methylbenzo[d][1,3]selenazole
CAS
2818-88-4
化学式
C8H7NSe
mdl
MFCD00005557
分子量
196.11
InChiKey
VYFYELQQECQPHU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:28.5-30 °C(lit.)
-
沸点:140 °C51 mm Hg(lit.)
-
闪点:>230 °F
-
稳定性/保质期:
避免与不相容材料接触,特别是强氧化剂。
计算性质
-
辛醇/水分配系数(LogP):1.92
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:6.1(b)
-
危险品标志:T,N
-
危险类别码:R50/53,R23/25,R33
-
危险品运输编号:UN 3077 9/PG 3
-
WGK Germany:3
-
RTECS号:DK7700000
-
海关编码:2927000090
-
包装等级:III
-
危险类别:6.1(b)
-
安全说明:S20/21,S28,S28A,S45,S60,S61
-
储存条件:密封保存,应储存在阴凉干燥的仓库中。
SDS
| Name: | 2-Methylbenzselenazole 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2818-88-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2818-88-4 | 2-METHYLBENZSELENAZOLE | 220-577-4 |
Risk Phrases: 23/25 33
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation and if swallowed. Danger of cumulative effects.
Potential Health Effects
Eye:
Causes mild eye irritation.
Skin:
Causes mild skin irritation.
Ingestion:
Poison by ingestion.
Inhalation:
The toxicological properties of this substance have not been fully investigated. Toxic if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid immediately. Wash mouth out with water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions.
Section 7 - HANDLING and STORAGE
Handling:
Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2818-88-4: United Kingdom, WEL - TWA: (listed as selenium compounds): 0.1 mg TWA (except hydrogen selenide, as Se) United Kingdom, WEL - STEL: (listed as selenium compounds): 0.3 m STEL (except hydrogen selenide, as Se) United States OSHA: 0.2 mg/m3 TWA (as Se) (listed under Selenium compounds).
Belgium - TWA: (listed as selenium compounds): 0.2 mg/m3 VLE (as Japan: (listed as selenium compounds): 0.1 mg/m3 OEL (except SeH2 SeF6, as Se) Malaysia: (listed as selenium compounds): 0.2 mg/m3 TWA (as Se) Netherlands: (listed as selenium compounds): 0.1 mg/m3 MAC (as Se Spain: (listed as selenium compounds): 0.1 mg/m3 VLA-ED (except hydrogen selenide, as Se) Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 140 deg C @ 51.00mm Hg
Freezing/Melting Point: 28.5 - 30 deg C
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H7NSe
Molecular Weight: 196.11
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide, selenium/selenium oxides.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2818-88-4: DK7700000 LD50/LC50:
CAS# 2818-88-4: Draize test, rabbit, eye: 500 mg/24H Mild; Draize test, rabbit, skin: 500 mg/24H Mild; Oral, rat: LD50 = 471 mg/kg.
Carcinogenicity:
2-METHYLBENZSELENAZOLE - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: SELENIUM COMPOUND, N.O.S.
Hazard Class: 6.1
UN Number: 3283
Packing Group: III
IMO
Shipping Name: SELENIUM COMPOUND, N.O.S.
Hazard Class: 6.1
UN Number: 3283
Packing Group: III
RID/ADR
Shipping Name: SELENIUM COMPOUND, N.O.S.
Hazard Class: 6.1
UN Number: 3283
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 23/25 Toxic by inhalation and if swallowed.
R 33 Danger of cumulative effects.
Safety Phrases:
S 20/21 When using do not eat, drink or smoke.
S 28A After contact with skin, wash immediately with
plenty of water.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2818-88-4: No information available.
Canada
CAS# 2818-88-4 is listed on Canada's NDSL List.
CAS# 2818-88-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2818-88-4 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质:无色或微黄色的固体。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-benzoselenazol-2-yl-propan-2-one 62693-30-5 C10H9NOSe 238.148
反应信息
-
作为反应物:描述:2-甲基-硒氮茚 在 三乙胺 作用下, 以 乙腈 为溶剂, 反应 7.08h, 生成 2-(3-methyl-3H-benzoselenazol-2-ylidene)-1-phenyl-ethanone参考文献:名称:Ciurdaru,G.; Ciuciu,M., Journal fur praktische Chemie (Leipzig 1954), 1979, vol. 321, p. 320 - 322摘要:DOI:
-
作为产物:描述:参考文献:名称:通过微波辅助方法从相应的N-(乙酰基)苯甲酰基-2-碘苯胺一锅制备2-(烷基)芳基苯并硒醇摘要:我们在这里报告从N-(乙酰基)苯甲酰基-2-碘苯胺单锅合成2-(烷基)芳基苯并硒唑的第一个例子。该反应在Woollins试剂的存在下在微波辐射下进行,并产生中等至良好的产率。DOI:10.1016/j.tetlet.2014.07.055
-
作为试剂:参考文献:名称:N-杂环与环氧丙烷和环氧氯丙烷的季铵化摘要:摘要 在高氯酸或氢溴酸存在下,环氧丙烷和环氧氯丙烷与适当的杂环碱反应,制备了几种新型重要的杂环 N-2-羟丙基和 N-2-羟基-3-氯丙基季盐,得到无色纯化合物。副产品。反应产物在 2 位或 4 位具有活化的甲基,是典型的前体,适用于合成不同类型的花青染料。所有产品均通过 1H-NMR 光谱和元素分析进行表征。DOI:10.1081/scc-200025601
文献信息
-
Symmetric Meso-Chloro-Substituted Pentamethine Cyanine Dyes Containing Benzothiazolyl/Benzoselenazolyl Chromophores Novel Synthetic Approach and Studies on Photophysical Properties upon Interaction with bio-Objects作者:Atanas Kurutos、Olga Ryzhova、Valeriya Trusova、Galyna Gorbenko、Nikolay Gadjev、Todor DeligeorgievDOI:10.1007/s10895-015-1700-4日期:2016.1A series of symmetric pentamethine cyanine dyes derived from various N-substituted benzothiazolium/benzoselenazolium salts, and a conjugated bis-aniline derivative containing a chlorine atom at meso-position with respect to the polymethine chain, were synthesized using a novel improved synthetic approach under mild conditions at room temperature. The reaction procedure was held by grinding the starting
-
Novel benzyne additions to the 1,2,5-thiadiazole and 1,2,5-selenadiazole ring systems作者:Martin R. Bryce、Peter Hanson、John M. VernonDOI:10.1039/c39820000299日期:——Novel Modes of benzyne addition to 3,4-dimethyl-1,2,5-thiadiazole and 3,4-dimethyl-1,2,5-selenadiazole afford methyl derivatives of three heterocyclic systems: quinoxaline, 1,2-benzisothiazole, and 1,3-benzoselenazole respectively.
-
Reactivity of heterocyclic nitrogen donors in systems containing the tetrachloroaurate(III) anion作者:Luciano Canovese、Lucio Cattalini、Michele Tomaselli、Martin L. TobeDOI:10.1039/dt9910000307日期:——same log K2versus pKa curve. There is no significant systematic steric effect on the equilibrium constants of the sort found for the more basic methyl pyridines. The complexes of the five-membered heterocyclic ligands are approximately ten times less reactive than those of the six-membered N-heterocycles of comparable basicity and exhibit steric retardation from ortho-methyl substituents. The nucleophilicities已经制备并表征了一系列[AuCl 3(L)]型的金(III)配合物(L =恶唑,苯并恶唑,噻唑,它们的苯并和甲基取代的衍生物或2-甲基苯并硒唑)。五元的N,O-N,S-和N,Se-杂环碱都通过氮与Au III结合。已在25.0°C和I = 0.20 mol dm –3(LiClO 4)的甲醇-水(95:5,v / v)中研究了氯化物置换L生成[AuCl 4 ] –的动力学。)。还已经确定了可逆过程的平衡常数。相应的吡啶,4-氯-,4-氰基-和2,6-双(氯甲基)-吡啶配合物的反应也已在相同条件下重新检查。平衡常数K 2取决于配体中氮的碱性,所有配体的点均与环的大小和组成无关,大致取决于对数K 2与p K a的关系。曲线。对于更碱性的甲基吡啶,对那种平衡常数没有明显的系统空间效应。五元杂环配体的配合物的反应性比具有相当碱性的六元N-杂环的配合物低约十倍,并且显示出由邻甲基取代基引起的空间阻滞
-
Polymethinfarbstoffe durch Ringspaltung des s-Triazins作者:A. KreutzbergerDOI:10.1002/ardp.19662991102日期:——dabei entstehenden Bruchstücke können gleichzeitig zum Ausgangspunkt neuer Synthesen werden. Die Einwirkung von quartären Methyl‐heterocyclen als nucleophile Reaktionskomponente auf s‐Triazin führt zu Polymethinfarbstoffen. Die neuartige Synthese dieser Farbstoffklasse ist mit aktiven Methylverbindungen aus dem Gebiete der Indole (X), Oxazole (XIX), Benzoxazole (XII), Benzthiazole (XIII, XXII) und Benzselenazole
-
Synthesis and Spectroscopic Characterisation of N-Alkyl Quaternary Ammonium Salts Typical Precursors of Cyanines作者:A. Pardal、S. Ramos、P. Santos、L. Reis、P. AlmeidaDOI:10.3390/70300320日期:——The synthesis and spectroscopic characterisation of some representative N-alkyl-substituted quaternary ammonium salts derived from benzothiazole, benzoxazole, benzo-selenazole, indole and quinoline are described. These heterocyclic salts, bearing an activated methyl group in the 2-position in relation to the nitrogen atom and N-methyl, -pentyl, -hexyl and -decyl chains, are typical precursors of cyanine
表征谱图
-
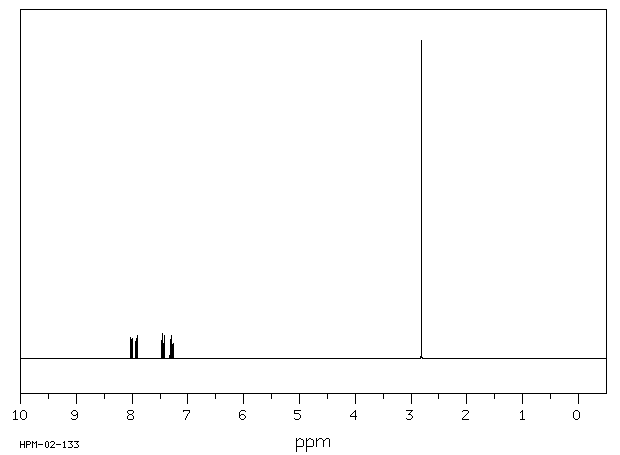
氢谱1HNMR
-
质谱MS
-
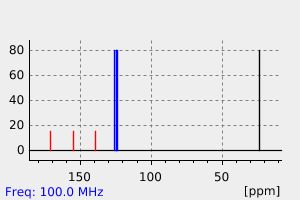
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-(+)-5,5'',6,6'',7,7'',8,8''-八氢-3,3''-二叔丁基-1,1''-二-2-萘酚,双钾盐
(S)-盐酸沙丁胺醇
(S)-溴烯醇内酯
(S)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2-N-Fmoc-氨基甲基吡咯烷盐酸盐
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(6,6)-苯基-C61己酸甲酯
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,5R)-3,3a,8,8a-四氢茚并[1,2-d]-1,2,3-氧杂噻唑-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aS,8aR)-2-(吡啶-2-基)-8,8a-二氢-3aH-茚并[1,2-d]恶唑
(3aS,3''aS,8aR,8''aR)-2,2''-环戊二烯双[3a,8a-二氢-8H-茚并[1,2-d]恶唑]
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(3-三苯基甲氨基甲基)吡啶
(3-[(E)-1-氰基-2-乙氧基-2-hydroxyethenyl]-1-氧代-1H-茚-2-甲酰胺)
(2′′-甲基氨基-1,1′′-联苯-2-基)甲烷磺酰基铝(II)二聚体
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,4S)-Fmoc-4-三氟甲基吡咯烷-2-羧酸
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环