5-Isobutyl-5-methyl-1,3-dichlor-hydantoin | 43161-90-6
中文名称
——
中文别名
——
英文名称
5-Isobutyl-5-methyl-1,3-dichlor-hydantoin
英文别名
1,3-dichloro-5-isobutyl-5-methyl-imidazolidine-2,4-dione;1,3-Dichlor-5-isobutyl-5-methyl-imidazolidin-2,4-dion;1,3-dichloro-5-methyl-5-isobutylhydantoin;1,3-Dichloro-5-methyl-5-(2-methylpropyl)imidazolidine-2,4-dione
CAS
43161-90-6
化学式
C8H12Cl2N2O2
mdl
——
分子量
239.101
InChiKey
CTKZXPQQBVOAGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:14
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:40.6
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 氯 、 sodium carbonate 作用下, 生成 5-Isobutyl-5-methyl-1,3-dichlor-hydantoin参考文献:名称:Okada et al., Yuki Gosei Kagaku Kyokaishi, 1957, vol. 15, p. 295,297,298摘要:DOI:
文献信息
-
COMPOSITIONS, METHODS, AND TEST KITS FOR DETERMINING AUTHENTICITY申请人:Milliken & Company公开号:US20190112484A1公开(公告)日:2019-04-18The present disclosure provides composition having at least one leuco composition conforming to Formula (I): Ar 1 Ar 2 Ar 3 CH (I) wherein Ar 2 and Ar 3 are independently a carbocyclic aryl or heteroaryl, and Ar 1 is selected from the group consisting of: unsubstituted phenyl, electron deficient carbocyclic aryl, and heteroaryl. The present disclosure also provides a method of detecting an authentic composition and test kits for detecting authentic compositions.本公开提供了一种具有至少一种符合以下式(I)的白色组合物的组合物:Ar1Ar2Ar3CH(I),其中Ar2和Ar3分别是一个碳环芳基或杂环芳基,而Ar1选自以下组:未取代的苯基、电子亏化的碳环芳基和杂环芳基。本公开还提供了一种检测真品组合物的方法以及用于检测真品组合物的检测试剂盒。
-
LAUNDRY CARE COMPOSITIONS, METHODS, AND TEST KITS FOR DETERMINING AUTHENTICITY申请人:The Procter & Gamble Company公开号:US20190112551A1公开(公告)日:2019-04-18The present disclosure provides a laundry care composition having at least one laundry care ingredient and at least one leuco composition conforming to Formula (I): Ar 1 Ar 2 Ar 3 CH (I) wherein Ar 2 and Ar 3 are independently a carbocyclic aryl or heteroaryl, and Ar 1 is selected from the group consisting of: unsubstituted phenyl, electron deficient carbocyclic aryl, and heteroaryl. The present disclosure also provides a method of determining an authentic laundry care composition and test kits for detecting authentic laundry care compositions.本公开提供一种洗涤护理组合物,其中至少包含一种洗涤护理成分和至少一种符合以下式(I)的还原组合物:Ar1Ar2Ar3CH (I)其中Ar2和Ar3分别是一个碳环芳基或杂环芳基,Ar1选自以下组:未取代的苯基、电子亏缺的碳环芳基和杂环芳基。本公开还提供了一种确定真正洗涤护理组合物的方法和用于检测真正洗涤护理组合物的测试套装。
-
Microbiological control in aqueous systems申请人:——公开号:US20030228341A1公开(公告)日:2003-12-11Biocidally-active 1,3-dibromo-5,5-dialkylhydantoin biocidal compositions in readily identifiable forms are provided in the form of an article of manufacture comprising a packaging material and a biocidal composition contained within said packaging material, wherein said biocidal composition comprises as the biocidally-active component of the biocidal composition (A) at least one 1,3-dibromo-5,5-dialkylhydantoin in which one of the alkyl groups in the 5-position is a methyl group and the other alkyl group in the 5-position contains in the range of 1 to 4 carbon atoms (DBDAH) as the sole biocidally-active ingredient of the composition, or (B) a combination of (i) DBDAH and (ii) at least one non-fluid biocidally-active water treating agent compatible with DBDAH, wherein the weight ratio of (i) to (ii) is in the range of 0.1:0.4 to 99.9:0.1, preferably in the range of 1:1 to 99.9:0.1, more preferably in the range of 9:1 to 99.9:0.1, and most preferably in the range of 19:1 to 99.9:0.1. Each such category of biocidally-active component or components can be, and preferably is in admixture with from 0 to 90 wt % of biocidally-inactive components such as for example one or more binders, fillers, excipients, dyes or colorants, perfumes, stabilizers, and/or manufacturing by-products. The packaging material comprises at least a label suitably identifying the name of the product in the package, and at least a sticker identifying the contents as being an oxidizing agent. The label or another label associated with the packaging material preferably contains other specified information.提供了具有生物杀灭活性的1,3-二溴-5,5-二烷基海因醇生物杀灭组合物,其以易于识别的形式呈现为一种制品,包括包装材料和包含在该包装材料中的生物杀灭组合物,其中所述生物杀灭组合物包括作为生物杀灭组合物的生物杀灭活性成分(A)至少一种1,3-二溴-5,5-二烷基海因醇,其中5位的烷基中的一个是甲基,另一个烷基含有1至4个碳原子(DBDAH)作为该组合物的唯一生物杀灭活性成分,或者(B)由(i) DBDAH和(ii)至少一种与DBDAH兼容的非流体生物杀灭水处理剂的组合物组成,其中(i)与(ii)的重量比在0.1:0.4至99.9:0.1的范围内,优选在1:1至99.9:0.1的范围内,更优选在9:1至99.9:0.1的范围内,最优选在19:1至99.9:0.1的范围内。每种生物杀灭活性成分类别或组合物可以与0至90重量%的生物杀灭非活性成分混合,例如一个或多个粘合剂、填充剂、赋形剂、染料或着色剂、香料、稳定剂和/或制造副产品。包装材料至少包括一个标签,适当地标识包装中产品的名称,并且至少包括一个贴纸,标识内容为氧化剂。标签或与包装材料相关的另一个标签最好包含其他指定信息。
-
Application of ambient temperature cured polymers of prepolymers to a cured elastomer申请人:THE FIRESTONE TIRE & RUBBER COMPANY公开号:EP0123070A2公开(公告)日:1984-10-31A process and composition for applying and bonding an amine curable polymer or prepolymer at ambient temperatures to the surface of a cured elastomer substrate. The elastomer article or substrate is treated with an organic oxidant such as an N-halohydantoin, N-haloamide, or an N-haioimide, for example, the various isomers of mono-, di-, and trichloroisocyanuric acid. A polyisocyanate is applied thereto. A composition of the amine curable polymer or prepolymer is prepared with an amine curing agent. To the curing agent and curable polymer or prepolymer composition is added a polar solvent which is mixed with the resulting blend or mixture and added to the treated elastomer surface; said polymer or prepolymer cures at ambient temperature and becomes bonded to said elastomer. The application is relatively easy, since the entire mixture is a liquid and can be applied in situ. The invention is particularly suitable for repairing various damaged elastomer articles such as tires (especially off-the-road), conveyor belts, hoses, preparation of caulking or sealing compounds, and especially in bonding tire lugs or tire treads to a tire carcass.一种在环境温度下将胺固化聚合物或预聚物涂抹和粘合到固化弹性体基材表面的工艺和组合物。用有机氧化剂,如 N-卤代海因、N-卤代酰胺或 N-卤代亚胺,例如一氯、二氯和三氯异氰尿酸的各种异构体,处理弹性体制品或基材。多异氰酸酯用于其中。用胺固化剂制备胺固化聚合物或预聚物的组合物。在固化剂和可固化聚合物或预聚物组合物中加入极性溶剂,将其与所得混合物或混合料混合并加入处理过的弹性体表面;所述聚合物或预聚物在环境温度下固化并与所述弹性体粘合。由于整个混合物都是液体,可以就地使用,因此使用起来相对容易。本发明特别适用于修复各种受损的弹性体,如轮胎(尤其是越野轮胎)、传送带、软管,制备填缝剂或密封化合物,尤其适用于将胎耳或胎面粘合到轮胎胎体上。
-
Ambient temperature repair of elastomeric articles having a hollow or deformity therein申请人:THE FIRESTONE TIRE & RUBBER COMPANY公开号:EP0122480A1公开(公告)日:1984-10-24The repair of a reinforced elastomer article (10) having a hollow (12) therein relates to utilizing an amine curable polymer or prepolymer and a cured elastomer patch. The hollow (12) in the elastomer as well as the areas juxtaposition to the patch (20) is coated with a treating agent (30). The amine curable polymer or prepolymer (40) which may have rubber particles therein is then applied to the hollow as well as to the area between the patch and the elastomer and cured at ambient temperature. Alternatively, gum rubber is directly applied to the hollow and also to the treated patch area. One area of use is in the repair of tires.对具有中空(12)的加固弹性体制品(10)的修复涉及到胺固化聚合物或预聚物和固化弹性体补丁的使用。 弹性体中空(12)以及与补丁(20)并列的区域涂有处理剂(30)。 然后,将胺固化聚合物或预聚物(40)(其中可能含有橡胶颗粒)涂抹到中空以及补丁和弹性体之间的区域,并在环境温度下固化。 或者,将胶橡胶直接涂抹到中空以及处理过的补丁区域。 一个使用领域是轮胎的修复。
表征谱图
-
氢谱1HNMR
-
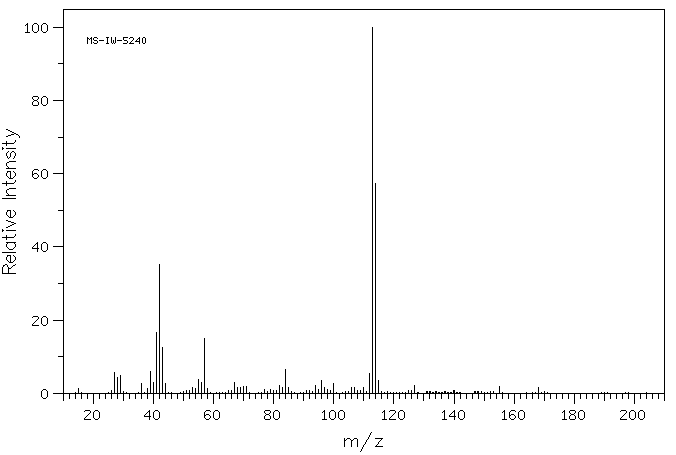
质谱MS
-
碳谱13CNMR
-
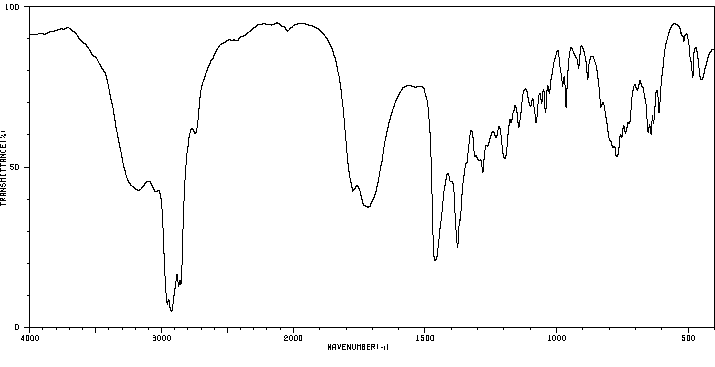
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-4-异丙基-2-恶唑烷硫酮
麻黄恶碱
顺-八氢-2H-苯并咪唑-2-酮
顺-1-(4-氟苯基)-4-[1-(4-氟苯基)-4-羰基-1,3,8-三氮杂螺[4.5]癸-8-基]环己甲腈
非达司他
降冰片烯缩醛3-((1S,2S,4S)-双环[2.2.1]庚-5-烯-2-羰基)恶唑烷-2-酮
阿齐利特
阿那昔酮
阿洛双酮
阿帕鲁胺
阿帕他胺杂质2
铟烷-2-YL-甲基胺盐酸
钾3-{2-[3-氰基-3-(十二烷基磺酰基)-2-丙烯-1-亚基]-1,3-噻唑烷-3-基}-1-丙烷磺酸酯
钠2-{[4,5-二羟基-3-(羟基甲基)-2-氧代-1-咪唑烷基]甲氧基}乙烷磺酸酯
重氮烷基脲
詹氏催化剂
解草恶唑
解草噁唑
表告依春
螺莫司汀
螺立林
螺海因氮丙啶
螺[咪唑烷-4,3'-吲哚啉]-2,2',5-三酮
螺[1-氮杂双环[2.2.2]辛烷-8,5'-咪唑烷]-2',4'-二酮
苯甲酸,4-氟-,2-[5,7-二(三氟甲基)-1,8-二氮杂萘-2-基]-2-甲基酰肼
苯氰二硫酸,1-氰基-1-甲基-4-氧代-4-(2-硫代-3-噻唑烷基)丁酯
苯妥英钠杂质8
苯妥英钠
苯妥英-D10
苯妥英
苯基硫代海因半胱氨酸钠盐
苯基硫代乙内酰脲-谷氨酸
苯基硫代乙内酰脲-蛋氨酸
苯基硫代乙内酰脲-苯丙氨酸
苯基硫代乙内酰脲-色氨酸
苯基硫代乙内酰脲-脯氨酸
苯基硫代乙内酰脲-缬氨酸
苯基硫代乙内酰脲-异亮氨酸
苯基硫代乙内酰脲-天冬氨酸
苯基硫代乙内酰脲-亮氨酸
苯基硫代乙内酰脲-丙氨酸
苯基硫代乙内酰脲-D-苏氨酸
苯基硫代乙内酰脲-(NΕ-苯基硫代氨基甲酰)-赖氨酸
苯基乙内酰脲-甘氨酸
苏氨酸-1-(苯基硫基)-2,4-咪唑烷二酮(1:1)
色氨酸标准品002
膦酸,(2-羰基-1-咪唑烷基)-,二(1-甲基乙基)酯
脱氢-1,3-二甲基尿囊素
脱氢-1,3,8-三甲基尿囊素
聚(d(A-T)铯)