三(2-甲基烯丙基)胺 | 6321-40-0
中文名称
三(2-甲基烯丙基)胺
中文别名
——
英文名称
tris(2-methylallyl)amine
英文别名
trimethallylamine;trimethallyl-amine;Trimethallyl-amin;Trimethallylamin;2-methyl-N,N-bis(2-methylprop-2-enyl)prop-2-en-1-amine
CAS
6321-40-0
化学式
C12H21N
mdl
MFCD00008596
分子量
179.305
InChiKey
FXBJYRVIFGLPBC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:83-85 °C15 mm Hg(lit.)
-
密度:0.794 g/mL at 25 °C(lit.)
-
闪点:128 °F
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:13
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:3.2
-
危险品标志:Xi
-
安全说明:S16,S24/25
-
危险类别码:R10,R36/37/38
-
海关编码:2921199090
-
包装等级:III
-
危险类别:3.2
-
危险品运输编号:UN 1993 3/PG 3
SDS
| Name: | Tris-(2-Methylallyl)-Amine 99% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 6321-40-0 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6321-40-0 | Tris-(2-methylallyl)-Amine | 99 | 228-674-3 |
Risk Phrases: 10 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin irritation. May cause irritation and dermatitis. May cause cyanosis of the extremities.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated. Ingestion of large amounts may cause CNS depression.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Aspiration may lead to pulmonary edema. Vapors may cause dizziness or suffocation. Can produce delayed pulmonary edema. May cause burning sensation in the chest.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Water may be ineffective. Material is lighter than water and a fire may be spread by the use of water. Containers may explode in the heat of a fire. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 6321-40-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: pale-yellow
Odor: amine-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 83- 85 deg C @ 15.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 53 deg C ( 127.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .7940g/cm3
Molecular Formula: C12H21N
Molecular Weight: 179.30
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Acids, acid chlorides, acid anhydrides, carbon dioxide, strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6321-40-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Tris-(2-methylallyl)-Amine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: II
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.2
UN Number: 1993
Packing Group: II
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 10 Flammable.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 33 Take precautionary measures against static
discharges.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 6321-40-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 6321-40-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6321-40-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基烯丙胺 bis(2-methylallyl)amine 35000-15-8 C8H15N 125.214
反应信息
-
作为反应物:描述:三(2-甲基烯丙基)胺 在 (bis(1,2-diphenylphosphino)ethane)(2,5-norbornadiene)rhodium(I) tetrafluoroborate 作用下, 以 四氢呋喃 为溶剂, 反应 18.0h, 以98%的产率得到triisobutenylamine参考文献:名称:Catalytic Asymmetric Hydrogen Migration of Allylamines摘要:本篇综述介绍了日本多个研究小组共同努力下近期开发的BINAP-Rh(I)催化不对称异构化前手性烯丙胺的一些制备方面和实际应用。此外,还讨论了新颖而复杂的反应机理。 1. 引言 2. 催化剂开发 2.1. 钴催化剂 2.2. 铑催化剂 2.3. 配体BINAP 3. 制备方面 3.1. 实验室规模的异构化 3.2. 工业过程 4. 底物与范围 4.1. 烯丙胺 4.2. 烯丙醇 5. 反应机理 5.1. 催化途径 5.2. 手性识别机制 5.3. 结论 6. 应用 6.1. 薄荷醇 6.2. 香茅醇 6.3. 香茅醛衍生物 6.4. 生育酚侧链 6.5. 灭虫脲DOI:10.1055/s-1991-26541
-
作为产物:描述:参考文献:名称:一步一步将尿素转化为叔胺摘要:DOI:10.1021/jo0011181
文献信息
-
Convergent Synthesis of Polynitrile and/or Polyamine Dendrimers through Hydroaminomethylation and Michael Addition作者:Maryam Beigi、Stefan Ricken、Kai Sven Müller、Fikret Koç、Peter EilbrachtDOI:10.1002/ejoc.201001307日期:2011.3A general concept for a versatile convergent synthesis of polynitrile and/or polyamine dendrimers has been developed by applying Vogtle's procedure in combination with the tandem hydroformylation/reductive amination sequence, known as hydroaminomethylation. In this approach, first Vogtle's procedure is used to generate the desired dendron, which can then be attached to different types of polyfunctionalized
-
An Efficient Synthesis of Benzimidazoles via Palladium-Catalyzed Amine Exchange Reaction from Trialkylamines to o-Phenylenediamine in an Aqueous Medium作者:Ngoc Thang Tran、Chan Sik ChoDOI:10.5012/bkcs.2012.33.12.4188日期:2012.12.20known as amine exchange reaction (amine scrambling reaction) and used for the synthesis of unsymmetrical amines and N-heterocycles and the study of the metabolism of amines. During the course of our studies directed towards transition metal-catalyzed C-N bond activation of alkylamines, we developed an alkyl (or alkanol) group transfer from alkylamines (or alkanolamines) to Natom of anilines as well过渡金属催化烷基胺之间的烷基转移被称为胺交换反应(amine scrambling reaction),用于不对称胺和N-杂环的合成以及胺代谢的研究。在我们针对过渡金属催化的烷基胺的 CN 键活化的研究过程中,我们开发了从烷基胺(或烷醇胺)到苯胺的 Natom 以及酮的 α-碳的烷基(或烷醇)基团转移,从而导致酮的区域选择性α-烷基化。在所采用的反应条件下,前一种转移最终导致吲哚和喹啉。然而,除了我们的发现之外,仅已知少数使用这种胺交换反应合成 N-杂环的例子。众所周知,氢嘧啶,咪唑烷和咪唑可以通过钯催化的二胺和烷基胺之间的分子间胺交换反应形成。发现二胺通过钌催化的分子内胺交换反应环化为吡咯烷、哌啶和氮杂环丙烷。另一方面,与本报告有关,Murahashi 等人。据报道,N-甲基苄胺与邻苯二胺在 Pd/C 存在下反应生成 2-苯基苯并咪唑和 1-苄基-2-苯基苯并咪唑,产率分别为 37% 和
-
Continuous hydrosilylation process申请人:Geisberger Gilbert公开号:US20060116525A1公开(公告)日:2006-06-01Continuous hydrosilylation of compounds (A) bearing C—C multiple bonds by means of silicon compounds (B) having Si—H groups, in which the reaction components (A) and (B) are reacted continuously in an integrated loop-tube reactor, with reaction mixture being conveyed from the tube into the loop and back again so that a section of the tube is part of the loop circuit, provides a highly controllable reaction process with high product yields.
-
Novel benzothiazepine and bensothiepine compounds申请人:Sasahara Takehiko公开号:US20070190041A1公开(公告)日:2007-08-16A pharmaceutical useful as a therapeutic agent and a preventive agent for hyperlipemia, and a pharmaceutical useful as a therapeutic agent and a preventive agent for hepatic disorders associated with cholestasis, particularly, primary biliary cirrhosis and primary sclerosing cholangitis, and a pharmaceutical useful as a therapeutic agent and a preventive agent for obesity, fatty liver and steatohepatitis are provided. A benzothiazepine or benzothiepine compound represented by the following formula (1A) having a thioamide bond and a quaternary ammonium substitutent:
-
Novel quaternary ammonium compounds申请人:Sasahara Takehiko公开号:US20070203115A1公开(公告)日:2007-08-30A method for inhibiting ileal bile acid transporter activity in a subject, comprising administering to said subject an effective amount of a compound represented by formula (1):一种抑制回肠胆汁酸转运蛋白活性的方法,包括向该受试者施用一种由式(1)所代表的化合物的有效量:
表征谱图
-
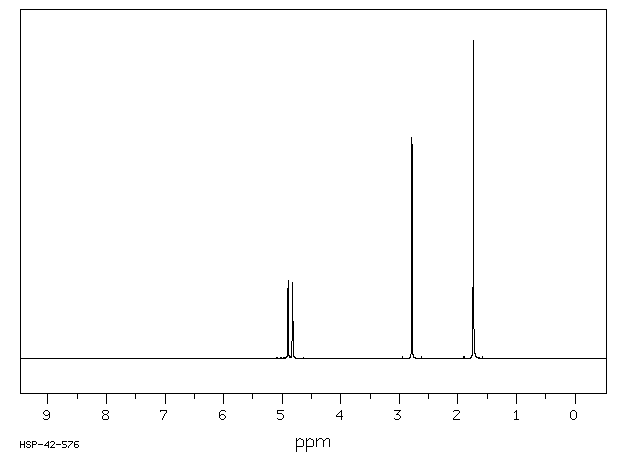
氢谱1HNMR
-
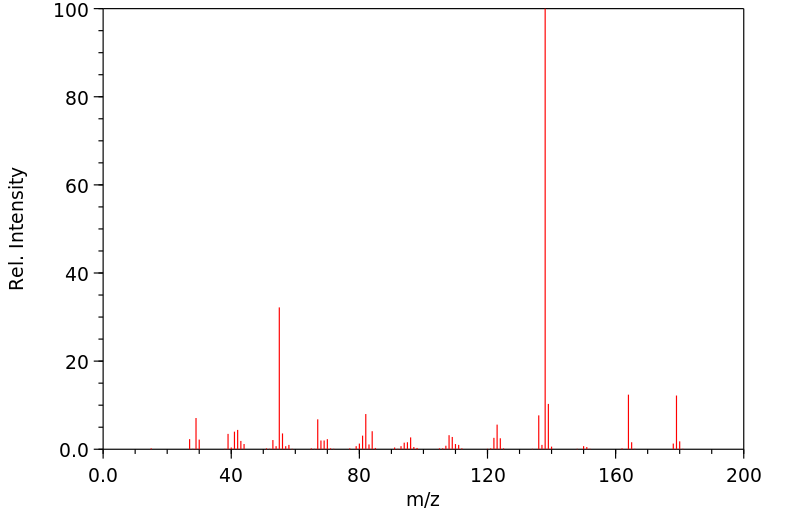
质谱MS
-
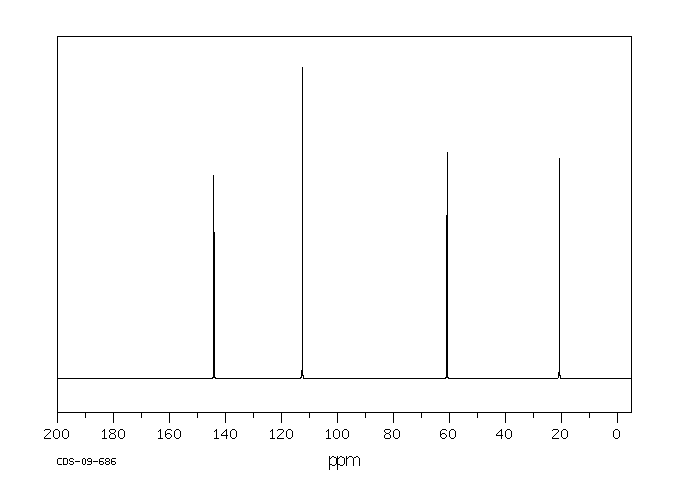
碳谱13CNMR
-
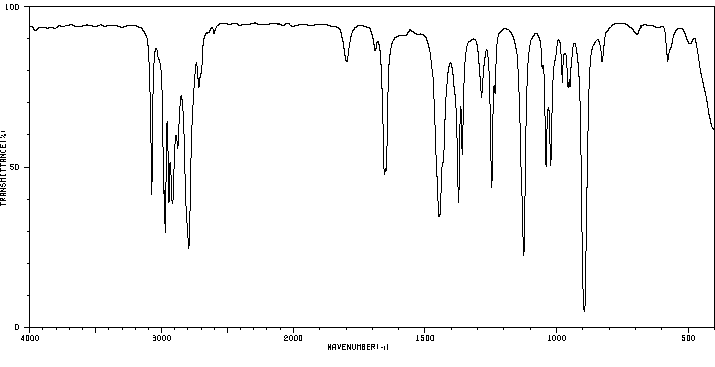
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷