二(乙硫基)乙酸乙酯 | 20461-95-4
中文名称
二(乙硫基)乙酸乙酯
中文别名
双(乙硫基)乙酸乙酯;双(乙基硫代)乙酸乙酯
英文名称
Bis(ethylthio)essigsaeure-ethylester
英文别名
ethyl 2,2-bis(ethylsulfanyl)acetate;ethyl glyoxylate diethyl thioacetal;ethyl bis(ethylthio)acetate;bis-ethylsulfanyl-acetic acid ethyl ester;Bis-aethylmercapto-essigsaeure-aethylester;Glyoxylic acid ethyl ester diethyl mercaptal
CAS
20461-95-4
化学式
C8H16O2S2
mdl
MFCD00009267
分子量
208.346
InChiKey
DGQHHBMWVOYSHU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:70 °C0.1 mm Hg(lit.)
-
密度:1.071 g/mL at 25 °C(lit.)
-
闪点:77 °F
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:12
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:76.9
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:3.2
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R10,R36/37/38
-
WGK Germany:3
-
海关编码:2930909090
-
包装等级:III
-
危险类别:3.2
-
危险品运输编号:UN 3272 3/PG 3
SDS
| Name: | Ethyl bis(ethylthio)acetate 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 20461-95-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 20461-95-4 | Ethyl bis(ethylthio)acetate, 99% | 99 | 243-836-3 |
Risk Phrases: 10
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
Flammable liquid and vapor.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep away from heat, sparks and flame. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 20461-95-4: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 70.0 - 70.0 deg C @ .10mm Hg
Freezing/Melting Point: 0 deg C
Autoignition Temperature: Not available.
Flash Point: 25 deg C ( 77.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.0710g/cm3
Molecular Formula: C8H16O2S2
Molecular Weight: 208.33
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 20461-95-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl bis(ethylthio)acetate, 99% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: II
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.2
UN Number: 1993
Packing Group: II
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
R 10 Flammable.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
WGK (Water Danger/Protection)
CAS# 20461-95-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 20461-95-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 20461-95-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Glyoxylsaeure-diaethylmercaptal 10490-06-9 C6H12O2S2 180.292
反应信息
-
作为反应物:描述:参考文献:名称:Bis (tributyltin) oxide. A mild, neutral and selective reagent for cleavage of esters. Scope and limitation of the reaction摘要:Bis(tributyltin)oxide acts as a mild, neutral and chemoselective ester cleavage reagent for a variety of carboxylic esters with good to excellent yields. The acyl-oxygen cleavage mechanism of (-)-(1R) menthyl acetate is discussed.DOI:10.1016/s0040-4039(00)92137-3
-
作为产物:描述:参考文献:名称:TAKEUCHI, YOSHIO;ASAHINA, MASAHIRO;HORI, KOZO;KOIZUMI, TORU, J. CHEM. SOC. PERKIN TRANS.,(1988) N 5, 1149-1153摘要:DOI:
文献信息
-
On Aromatic Electrophilic Substitution Promoted by In Situ Generated Thionium Ions作者:Liliana Marzorati、Rosana Amici、Claudio Di VittaDOI:10.1055/s-0032-1318167日期:——prepared via ethylthiomethylation reaction of aromatic compounds. These aromatic electrophilic substitutions were performed by in situ generation of the thionium ions by reacting trifluoroacetic anhydride and S-ethyl 2-(ethylsulfinyl)ethanethioate or ethyl 2-(ethylsulfinyl)acetate in the presence of Lewis acids. Some mechanistic features of these ethylthiomethylation reactions are also presented. α-Sulfanylated
-
3d-orbital resonance in divalent sulphide—X作者:S. Oae、W. Tagaki、K. Uneyama、I. MinamidaDOI:10.1016/s0040-4020(01)96324-0日期:1968.1Several α-mercaptoacetic and related acids were synthesized and the rates of the amine-catalysed decarboxylation were measured. In the carbanion-forming reaction, the α-mercapto group exhibits a dramatic rate-enhancement, due to the 3d-orbital resonance effect of the divalent sulphide group. For example, the rate of the decarboxylation of diphenylmercaptoacetic acid was 8·8 × 103 fold greater than that
-
Lissel, Manfred, Liebigs Annalen der Chemie, 1982, # 9, p. 1589 - 1601作者:Lissel, ManfredDOI:——日期:——
-
Scope and Mechanism of Deprotection of Carboxylic Esters by Bis(tributyltin) Oxide作者:Claudio J. Salomon、Ernesto G. Mata、Oreste A. MascarettiDOI:10.1021/jo00103a016日期:1994.12Methyl and ethyl esters of aliphatic and aromatic carboxylic acids as well as benzyl carboxylates, thiol esters and double esters such as (pivaloyloxy)methyl carboxylates have been successfully cleaved with bis(tributyltin) oxide to give the free carboxylic acids in good yields. The reaction is carried out in aprotic solvents under essentially neutral conditions and thus this method can serve as an ideal procedure for the cleavages of esters with other functional groups and/or protecting groups acid and/or base sensitive. We demonstrated that the reaction displays a high level of chemoselectivity between methyl and ethyl esters versus tert-butyl esters and gamma-lactones. Bis(tributyltin) oxide is also a highly efficient reagent for the cleavage of acetates of primary and secondary alcohols and phenols. The limitations we found in the use of this reagent include the lack. of cleavage of esters sterically hindered around the carboxyl carbon and the carbinol group (i.e., esters of tertiary alcohols) and in carboxylic esters that contain a fluoroalkyl substituent. A resonable mechanistic explanation is discussed to account for the reaction pathway of the acyloxygen cleavage of(-)-(1R)-menthyl acetate.
-
7α- and 17α-Substituted estrogens containing tridentate tricarbonyl rhenium/Technetium complexes: synthesis of estrogen receptor imaging agents and evaluation using microPET with technetium-94m作者:Leonard G. Luyt、Heather M. Bigott、Michael J. Welch、John A. KatzenellenbogenDOI:10.1016/j.bmc.2003.09.004日期:2003.11To develop technetium and rhenium-labeled imaging agents for estrogen receptor (ER) positive breast tumors, we have prepared tridentate metal tricarbonyl chelates substituted at the 7alpha- and 17alpha-positions of estradiol. Some of the Re(CO)(3) conjugates have high binding for the ER in vitro. The in vivo biodistribution of the highest affinity of these novel metal tricarbonyl conjugates, prepared as the Tc-94m labeled analogue, was evaluated by tissue dissection and microPET imaging. Although target tissue-selective uptake was not apparent, it is notable that microPET imaging identified the stomach as a major site of activity deposition, a site that might have been missed by standard tissue distribution studies. (C) 2003 Elsevier Ltd. All rights reserved.
表征谱图
-
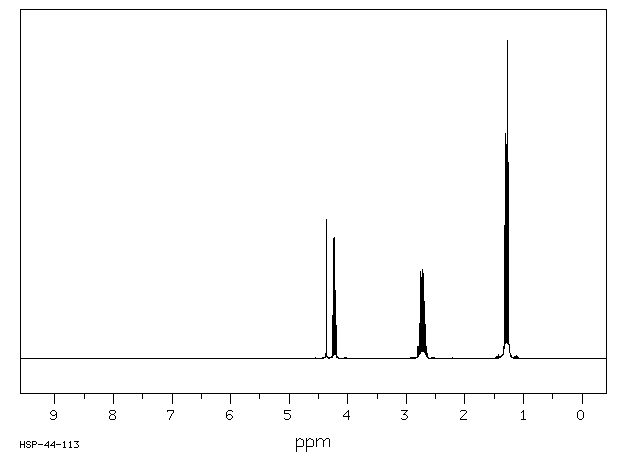
氢谱1HNMR
-
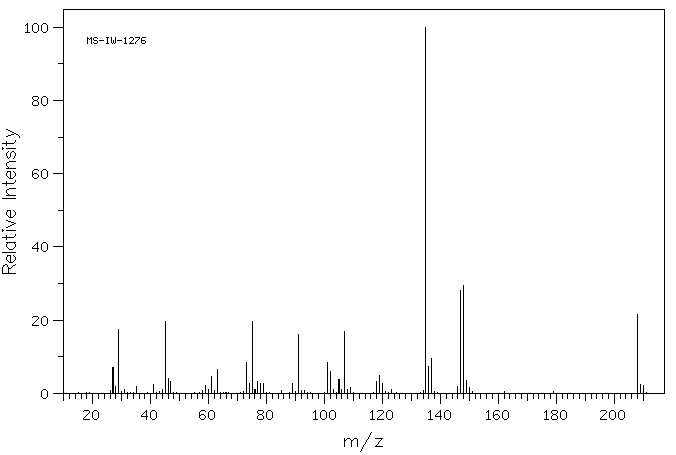
质谱MS
-
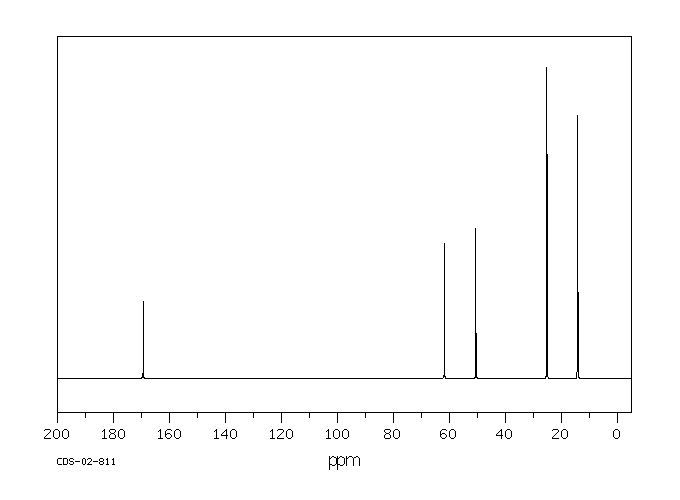
碳谱13CNMR
-
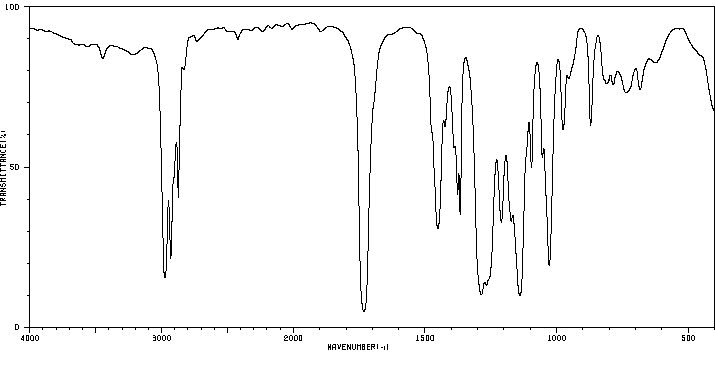
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
雷尼替丁EP杂质J
苯乙酮乙烷-1,2-二基二硫代缩醛
苯丙酮乙烷-1,2-二基二硫代缩醛
磷亚胺酸,[2,3,4,5,6-五氯-2,3,5,6-四氟-1-(2,2,3,3-四氟丙氧基)-4-(三氟甲基)环己基]-,三(2,2,3,3-四氟丙基)酯
硫代磷酸O,O-二乙基S-[2,2-二(乙硫基)丙基]酯
硫代二碳酸叔丁基乙基酯
硫代二碳酸 1-乙基 3-异丙基酯
甲硫基甲酸叔丁酯
甲氧基甲基硫烷基乙烷
甲氧基二硫代甲酸甲酯
甲氧基(甲基硫烷基)甲烷
甲基二[[(二甲基氨基)硫代甲酰]硫代]乙酸酯
甲基8-氧代-6,10-二硫杂螺[4.5]癸烷-7-羧酸酯
环辛酮硫代缩酮
环线威
环己基甲硫基甲基醚
环己基二乙酸二乙酯
氰硫基酸,2,2,2-三氯乙基酯
双(硫代甲氧基甲基)硫醚
双(亚甲基二硫代)四硫富瓦烯
六氢-2'3A-二甲基螺[1,3-二硫环戊并[4,5-B]呋喃-2,3'(2'H)-呋喃]
亚甲基二(氰基亚胺硫代碳酸甲酯)
亚甲基二(二异丁基二硫代氨基甲酸酯)
二邻茴香醚
二硫氰基甲烷
二硫代丁酸甲酯
二甲硫基甲烷
二甲氧基-[(2-甲基-1,3-氧硫杂环戊烷-2-基)甲硫基]-巯基膦烷
二异丙基黄原酸酯
二(硫代碳酸 O-丁基酯)硫代酸酐
二(二甲基二硫代氨基甲酸)亚甲基酯
二(乙硫基)甲烷
二(乙硫基)乙酸乙酯
二(乙氧基硫代羰基)硫醚
二(2-氨基乙基硫基)甲烷
乙醛,二(甲硫基)-
乙酸甲硫甲酯
乙氧基甲基异硫脲盐酸盐
乙丙二砜
乙丁二砜
丙烷-2、2-二基双(磺胺二基)二乙胺
丙烷-2,2-二基双(硫)基]二乙酸
三硫丙酮
[(异丙氧基硫基甲酰基硫基)硫基甲酰基硫基]硫代甲酸O-异丙基酯
[(N,N-二甲基二硫代氨基甲酰)甲基]甲基氰基亚氨二硫代碳酸酯
[(2R,4S,6R)-4,6-二甲基-1-硫羟基-1,3-二硫烷-2-基](二苯基)磷烷
[(2-羧基乙氧基)甲基]二甲基-锍溴化物(1:1)
S-甲基O-(2-甲基丙基)二硫代碳酸酯
S-烯丙基 O-戊基二硫代碳酸酯
S-乙基O-(1-碘乙基)硫代碳酸酯