二氯丙二腈 | 13063-43-9
中文名称
二氯丙二腈
中文别名
2,2-二氯丙二腈
英文名称
dichloromalononitrile
英文别名
dichloromalonitrile;2,2-dichloropropanedinitrile
CAS
13063-43-9
化学式
C3Cl2N2
mdl
MFCD00001841
分子量
134.952
InChiKey
NCWGPYPDPWOJKC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:97 °C(lit.)
-
密度:1.312 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
稳定性/保质期:
在常温常压下稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:7
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:47.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S26,S27,S37/39
-
危险类别码:R23/24/25
-
WGK Germany:3
-
海关编码:2926909090
-
危险品运输编号:UN 2810 6.1/PG 2
-
RTECS号:OO3740000
-
包装等级:III
-
危险类别:6.1
-
储存条件:常温下应存放在密闭容器中,并置于阴凉、通风和干燥处。
SDS
| Name: | Dichloromalononitrile 97% Material Safety Data Sheet |
| Synonym: | Malononitrile, dichloro |
| CAS: | 13063-43-9 |
Synonym:Malononitrile, dichloro
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 13063-43-9 | Dichloromalononitrile | 97 | 235-955-4 |
Risk Phrases: 23/24/25
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed.Lachrymator (substance which increases the flow of tears).Toxic.
Potential Health Effects
Eye:
Causes severe eye irritation. Lachrymator (substance which increases the flow of tears). May cause chemical conjunctivitis and corneal damage.
Skin:
Causes severe skin irritation.
Ingestion:
Harmful if swallowed. May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
May cause respiratory tract irritation. May cause burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do not use mouth-to-mouth resuscitation if victim ingested or inhaled the substance; induce artificial respiration with the aid of a pocket mask equipped with a one-way valve or other proper respiratory medical device.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Do not get in eyes, on skin, or on clothing. Do not ingest or inhale. Use only in a chemical fume hood. Do not breathe vapor.
Storage:
Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closed.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 13063-43-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Wear a NIOSH/MSHA or European Standard EN 149 approved full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: clear yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 97 deg C @ 760.00mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: > 112 deg C (> 233.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: 1.3120g/cm3
Molecular Formula: C3Cl2N2
Molecular Weight: 134.95
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, excess heat.
Incompatibilities with Other Materials:
Strong acids, strong bases, strong oxidizing agents, strong reducing agents.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide, chloride fumes.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 13063-43-9: OO3740000 LD50/LC50:
CAS# 13063-43-9: Draize test, rabbit, skin: 200 uL/24H Severe; Oral, rabbit: LD50 = >126 mg/kg; Oral, rat: LD50 = 130 mg/kg.
LD50: Oral, rat = 130 Carcinogenicity:
Dichloromalononitrile - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: II
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: II
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 13063-43-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 13063-43-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 13063-43-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:二氯丙二腈 在 fluorine 作用下, 生成 2,2-Dichlor-perfluor-(1,3-propandiamin)参考文献:名称:The Action of Elementary Fluorine upon Organic Compounds. XXVIII. The Direct Fluorination of Perhalonitriles摘要:DOI:10.1021/ja01063a034
-
作为产物:描述:参考文献:名称:Friedrich,K.; Rieser,J., Justus Liebigs Annalen der Chemie, 1976, p. 648 - 655摘要:DOI:
文献信息
-
Construction of carbocycles initiated by Cu-catalyzed radical reaction of Cl2C(CN)2作者:Keisuke Masuda、Masashi Tanigawa、Masanori Nagatomo、Daisuke Urabe、Masayuki InoueDOI:10.1016/j.tet.2017.03.063日期:2017.6A Cu-catalyzed radical reaction of Cl2C(CN)2 was utilized for stereoselective conversion of unsaturated molecules into functionalized carbocycles. Chloromalononitrile radicals, generated by treating Cl2C(CN)2 with catalytic amounts of CuCl and dppf in refluxing dioxane, intermolecularly reacted with the unsaturated bonds of acyclic or 10-membered compounds. The resultant carboradicals then intramolecularly
-
Attachment of carbonyl functionalities onto olefins via copper-promoted radical reaction of dichloromethylcyanides作者:Shin Kamijo、Shinya Yokosaka、Masayuki InoueDOI:10.1016/j.tet.2012.01.089日期:2012.7Chemo- and regioselective protocols for attachment of various carbonyl functionalities onto unactivated olefins have been developed. Atom transfer radical reactions of Cl3CCN, Cl2C(R)CN, and Cl2C(CN)2 were all promoted efficiently by a catalytic amount of CuCl and 1,1′-bis(diphenylphosphino)ferrocene to introduce chloromethylcyanide and chloride units to the C–C double bonds. Conversion of the chloromethylcyanide
-
Reactivity of Lewis acids towards nitriles; crystal structure and electron deformation density of C<sub>2</sub>N<sub>2</sub>·SbF<sub>5</sub>and photoelectron spectrum of AsF<sub>5</sub>作者:Inis C. Tornieporth-Oetting、Thomas M. Klapötke、T. Stanley Cameron、Jussi Valkonen、Paul Rademacher、Klaus KowskiDOI:10.1039/dt9920000537日期:——nitriles and dinitriles HCN, C2N2, CCl2(CN)2 and CH2(CN)2 towards AsF5 and SbF5 has been investigated. The new compounds HCN·AsF51, HCN·SbF52, C2N2·AsF53, C2N2·SbF54, CH2(CN)2·AsF55, CH2(CN)2·(2AsF5)6 and CCl2(CN)2·AsF57 have been characterized by chemical analysis, IR, Raman, 1H, 19F and 14N NMR spectroscopy. The crystal structure of the first example of a cyanogen adduct C2N2·SbF54 has been determined研究了腈和二腈HCN,C 2 N 2,CCl 2(CN)2和CH 2(CN)2对AsF 5和SbF 5的反应性。新化合物HCN·AsF 5 1,HCN·SbF 5 2,C 2 N 2 ·AsF 5 3,C 2 N 2 ·SbF 5 4,CH 2(CN)2 ·AsF 5 5,CH 2(CN)2 ·(2AsF 5)6和CCl 2(CN)2 ·AsF 5 7已通过化学分析,IR,拉曼,1 H,19 F和14 N NMR光谱进行了表征。已经确定了氰化物加合物C 2 N 2 ·SbF 5 4的第一实例的晶体结构。化合物4在正交晶空间群C 222 1中结晶,晶格参数为a = 6.579(1),b = 16.245(1)和c= 6.389(1)Å。检查了分子的变形密度。基于硬-软酸碱原理,讨论了HCN和C 2 N 2对强路易斯酸AsF 5的反应性。通过光电子能谱获得了迄今未知的AsF 5电离势(15.57
-
Sequential ATRA/Reductive Cyclopropanation Reactions with a Ruthenium Catalyst in the Presence of Manganese作者:Mariano A. Fernández-Zúmel、Charlotte Buron、Kay SeverinDOI:10.1002/ejoc.201100175日期:2011.4addition (ATRA) reactions of ethyl trichloroacetate, dichloromalononitrile, or diethyl 2,2-dichloromalonate with olefins followed by dechlorination have provided functionalized cyclopropanes in one step. The RuIII complex [Cp*RuCl2(PPh3)] was used as a catalyst precursor and commercial Mn powder as reducing agent. Reactions with the less activated substrate ethyl dichloroacetate gave ATRA products with
-
Ruthenium-Catalyzed Synthesis of 1,5-Dichlorides by Sequential Intermolecular Kharasch Reactions作者:Katrin Thommes、Mariano A. Fernández-Zúmel、Charlotte Buron、Aurélien Godinat、Rosario Scopelliti、Kay SeverinDOI:10.1002/ejoc.201001213日期:2011.1Sequential intermolecular atom transfer radical addition reactions of activated dichlorides Cl2CRR′ (R = CN, CO2Et, R′ = H, CN, CO2Et) with two olefins catalyzed by [Cp*RuCl2(PPh3)] in the presence Mg allow the synthesis of linear 1,5-dichlorides. Different olefins can be employed in the first and in the second addition reaction. The reaction products are interesting synthetic precursors as demonstrated
表征谱图
-
氢谱1HNMR
-
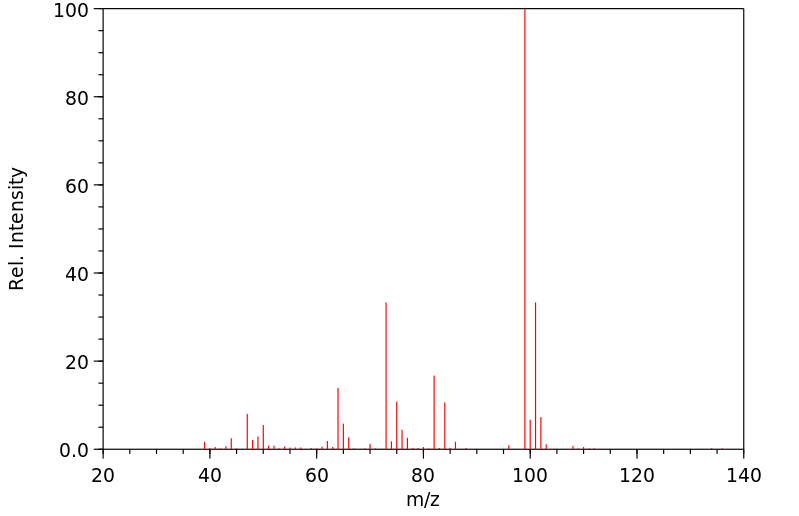
质谱MS
-
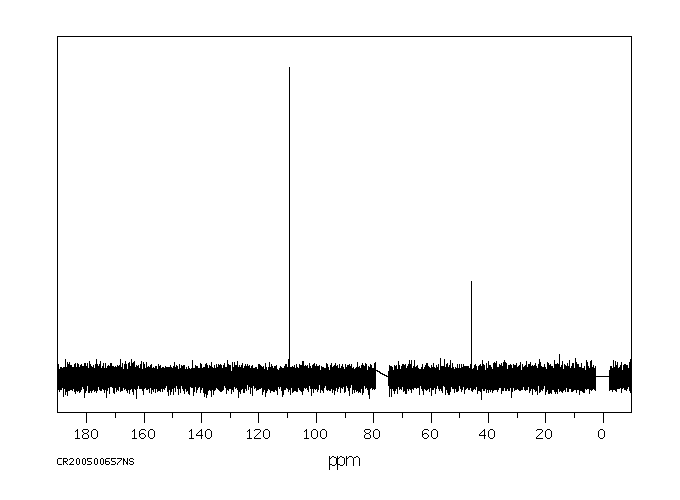
碳谱13CNMR
-
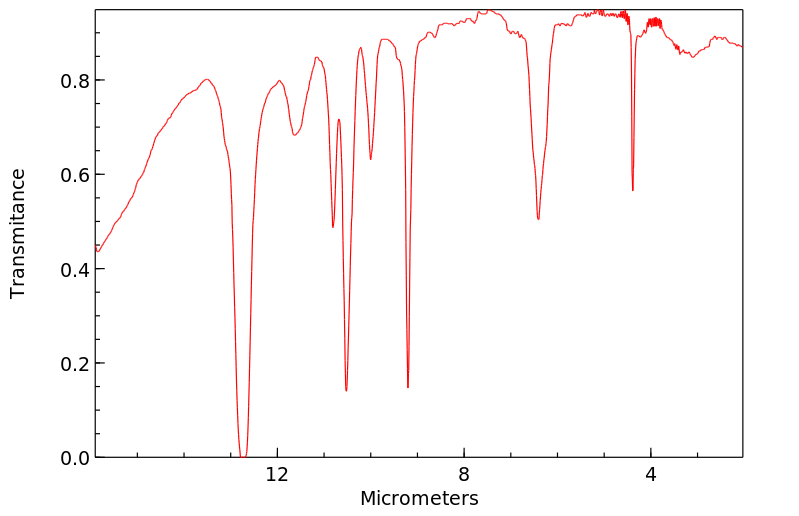
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷