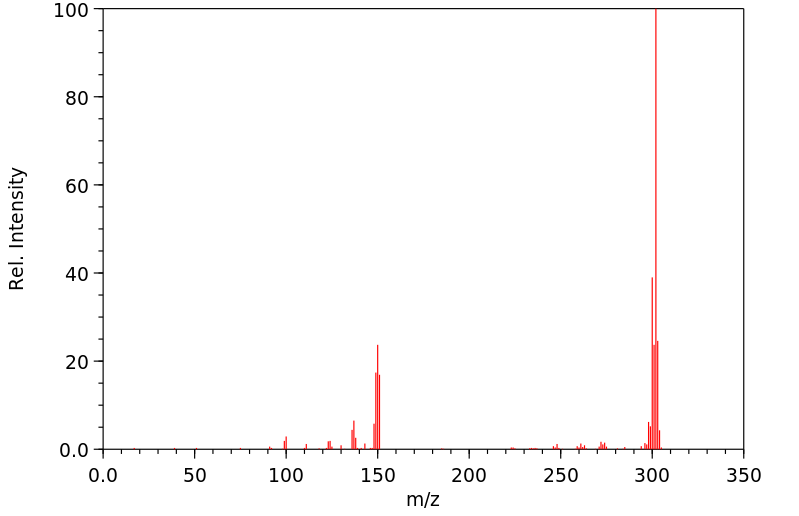
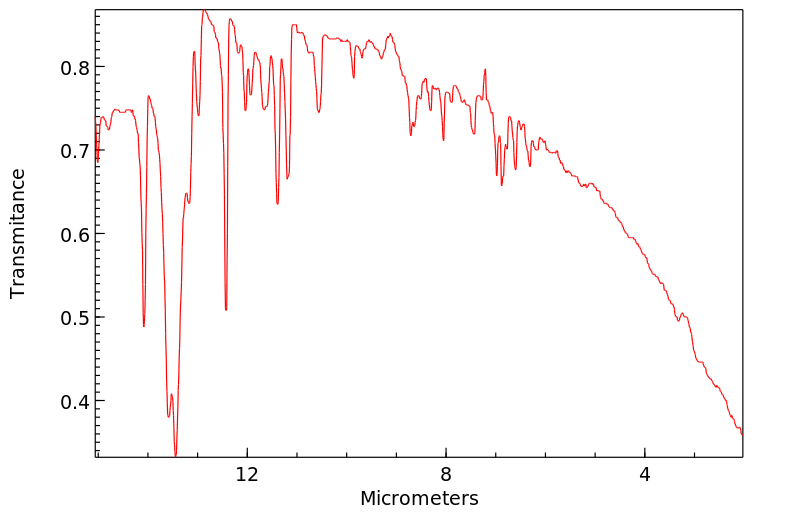
代谢
与其他多环芳烃一样,二苯并(a,l)芘需要代谢激活才能发挥其致突变和/或致癌活性。在人类乳腺癌细胞系MCF-7中,二苯并(a,l)芘立体选择性地代谢为(-)-反式和(+)-3-顺式-二苯并(a,l)芘-11,12-二醇13,14-环氧化物(DB(a,l)PDE),这两种物质都会大量结合到DNA中的脱氧腺苷残基上。为了进一步表征其强烈致癌性的潜在机制,研究了二苯并(a,l)芘的DNA结合与致突变性之间的关系。还研究了外消旋二苯并(a,l)芘-11,12-二氢二醇及其两个单独的(+)-和(-)-对映体,这是立体异构的峡湾区二氢二醇环氧化物的代谢前体。在MCF-7细胞介导的中国仓鼠V79细胞突变试验中,测量了HPRT位点的突变诱导。在试验条件下,亲本烃、(+或-)-二苯并(a,l)芘-11,12-二氢二醇和(-)-二苯并(a,l)芘-11,12-二氢二醇都具有高度致突变性。相比之下,使用MCF-7细胞作为代谢激活系统时,(+)-二苯并(a,l)芘-(11S,12S)-二氢二醇不具有致突变性。在相同的实验中分析DNA加合物时发现,用(-)-二苯并(a,l)芘-11,12-二氢二醇处理的MCF-7细胞形成了唯一的(-)-反式-DB(a,l)PDE加合物,而用(+)-二苯并(a,l)芘-11,12-二氢二醇处理的细胞中没有检测到DNA加合物的可检测水平。这些结果表明,特定的细胞色素p450酶可能对激活两种二苯并(a,l)芘-11,12-二氢二醇对映体具有高立体选择性,这可能在人类细胞中强烈致癌物二苯并(a,l)芘的代谢激活中发挥重要作用。
... Like other polycyclic aromatic hydrocarbons, dibenzol(a,l)pyrene requires metabolic activation to exert its mutagenic and/or carcinogenic activity. In the human mammary carcinoma cell line MCF-7, dibenzol(a,l)pyrene is stereoselectively metabolized to the (-)-anti- and (+)-3-syn-dibenzol(a,l)pyrene-11,12-diol 13,14-epoxides (DB(a,l)PDE) which both bind extensively to deoxyadenosine residues in DNA. To further characterize the underlying mechanism of its strong carcinogenicity, the relationship between DNA binding and mutagenicity of dibenzol(a,l)pyrene was determined. Racemic dibenzol(a,l)pyrene-11,12-dihydrodiol and the two individual (+)- and (-)-enantiomers, the metabolic precursors of the stereoisomeric fjord region dihydrodiol epoxides, were also investigated. Induction of mutations at the HPRT locus was measured in a MCF-7 cell-mediated Chinese hamster V79 cell mutation assay. The parent hydrocarbon, (+ or -)-dibenzol(a,l)pyrene-11,12-dihydrodiol, and (-)-dibenzol(a,l)pyrene-11,12-dihydrodiol were highly mutagenic under the assay conditions. In contrast, (+)-dibenzol(a,l)pyrene-(llS,12S)-dihydrodiol was not mutagenic using MCF-7 cells as the metabolic activating system. Analysis of DNA adducts in the same experiments revealed that MCF-7 cells treated with (-)-dibenzol(a,l)pyrene-11,12-dihydrodiol formed exclusively (-)-anti-DB(a,l)PDE adducts whereas cells treated with (+)-dibenzol(a,l)pyrene-11,12-dihydrodiol did not contain detectable levels of DNA adducts. These results suggest that specific cytochrome p450 enzymes may have high stereoselectivity for activation of the two dibenzol(a,l)pyrene-11,12-dihydrodiol enantiomers, and this may play an important role in the metabolic activation of the strong carcinogen dibenzol(a,l)pyrene in human cells.
来源:Hazardous Substances Data Bank (HSDB)