双(6-羟基-2-萘)二硫 | 6088-51-3
中文名称
双(6-羟基-2-萘)二硫
中文别名
2,2-二羟基二硫代-6,6-联萘;6,6'-二硫-2,2'-二萘酚;2,2ˊ-二羟基-6,6ˊ-二萘二硫;1,1-二氯-2,2-双(4-氯苯基)乙烷;2,2'-二氯二苯基二硫化物;6,6'-二羟基-2,2'-二萘基二硫醚;6,6-二硫-2,2-二萘酚
英文名称
6-hydroxy-2-naphthyldisulphide
英文别名
6-Hydroxy-2-naphthyldisulfide;6-Hydroxy-2-naphthyl disulfide;6-[(6-hydroxynaphthalen-2-yl)disulfanyl]naphthalen-2-ol
CAS
6088-51-3
化学式
C20H14O2S2
mdl
MFCD00004083
分子量
350.462
InChiKey
AHXGXXJEEHFHDK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:226 °C
-
沸点:597.5±25.0 °C(Predicted)
-
密度:1.46±0.1 g/cm3(Predicted)
-
溶解度:<26.28mg/ml,溶于 DMSO
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:24
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:91.1
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2930909090
-
安全说明:S26,S36
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Blokhin, A. P.; Gelin, M. F.; Kalosha, I. I., Journal of Applied Spectroscopy, 1999, vol. 66, # 1, p. 55 - 61摘要:DOI:
-
作为产物:描述:参考文献:名称:Effects of oxygen–sulfur substitution on glycosaminoglycan-priming naphthoxylosides摘要:Three series of sulfur-containing analogs to the selectively antiproliferative 2-(6-hydroxynaphthyl) beta-D-xylopyranoside were synthesized and their biological properties investigated. A short, general route to hydroxynaphthyl disulfides from dihydroxy-naphthalenes was developed to utilize the disulfide bond as a sulfur-selective protecting group to enable the orthogonal protection of hydroxyls and thiols. The results indicate that hydrophobic, uncharged oxygen-sulfur substituted naphthoxylosides are taken up by calls and initiate priming of GAG chains to a greater extent compared to the oxygen analogs. No correlation between priming ability and antiproliferative activity was observed. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2007.05.008
-
作为试剂:描述:参考文献:名称:Method for preparing mercaptopropionic acid esters摘要:提供了一种制备巯基丙酸酯的方法,该方法通过在弱碱性胺作为催化剂,聚醚作为协同催化剂,聚硫代丙酸酯作为反应溶剂,以及添加元素硫的情况下,将丙烯酸酯与氢硫化物反应。公开号:US05157147A1
文献信息
-
[EN] BORONATE-MEDIATED DELIVERY OF MOLECULES INTO CELLS<br/>[FR] ADMINISTRATION DE MÉDICAMENT MÉDIÉE PAR LES BORONATE申请人:WISCONSIN ALUMNI RES FOUND公开号:WO2013110005A1公开(公告)日:2013-07-25Methods for enhancing cellular uptake of cargo molecules by boronating the cargo molecule, particularly with one or more phenylboronic acid groups. Cellular uptake includes at least partial uptake into the cytosol. Boronation includes ligating, crosslinking or otherwise bonding one or more phenylboronic acids substituted to contain a reactive group to a cargo molecule. Boronation also includes ligating, crosslinking or otherwise bonding a phenylboronated oligopeptide to a cargo molecule. The phenylboronate groups are optionally conjugated to the cargo molecule via linking moieties that can be selectively cleaved. The invention includes certain phenylboronates which are boronation reagents, certain boronated oligopeptides and certain boronated peptides and proteins. The invention also includes kits for enhancing cellular uptake of cargo molecules by boronation with one or more phenylboronates or boronated oligopeptides.
-
CURATIVES FOR EPOXY ADHESIVE COMPOSITIONS申请人:Dershem Stephen M.公开号:US20100113643A1公开(公告)日:2010-05-06The invention provides epoxy and oxetane compositions including the novel acyloxy and N-acyl curing agents described herein. Use of invention curing agents result in cured adhesive compositions with remarkably increased adhesion and reduced hydrophilicity when compared to resins cured with other types of curing agents. Furthermore, the curatives of this invention do not interfere with free-radical cure and are thus suited for use in hybrid cure thermoset compositions.
-
[EN] DITHIOAMINE REDUCING AGENTS<br/>[FR] AGENTS RÉDUCTEURS DITHIOAMINE申请人:WISCONSIN ALUMNI RES FOUND公开号:WO2013123382A1公开(公告)日:2013-08-22Dithioamine reducing agents useful for the reduction of disulfide bonds. The reducing agents of this invention are useful, for example, to reduce disulfide bonds, particularly in proteins, or to prevent the formation of disulfide bonds, particularly in proteins and other biological molecules. Reducing agents of this invention can be employed to regulate protein function in proteins in which a sulfhydryl group is associated with biological activity. Reducing agents of this invention can prevent inactivation of a given protein or enhance activation of a given protein or other biological molecule in vitro and/or in vivo. Reducing agents of this invention can prevent or reduce oxidation of cysteine residues in proteins and prevent the formation of reduced activity protein dimers (or other oligomers). Reducing agents of this invention are useful and suitable for application in a variety of biological applications, particularly as research and synthetic reagents.
-
Switching catalytic reaction conducted in pore void of mesoporous material by redox gate control作者:Masahiro Fujiwara、Shigeki Terashima、Yasuko Endo、Kumi Shiokawa、Hiroyoshi OhueDOI:10.1039/b610444d日期:——A molecular gate attached on the pore outlet of a mesoporous material, which is opened and closed by redox system of thiol groups, effectively switched the progress of a catalytic reaction promoted by the acidic site in the pore void.一个分子门附着在介孔材料的孔口,通过硫醇基的氧化还原系统打开和关闭,有效地调控了由孔隙中的酸性位点促进的催化反应进程。
-
Brønsted acid-catalyzed aromatic annulation of alkoxyallenes with naphthols: a reaction sequence to larger π-conjugated naphthopyrans with aggregation-induced emission characters作者:Jinlong Zhang、Lu Zhu、Kang Shen、Huameng Yang、Xiao-Chun Hang、Gaoxi JiangDOI:10.1039/c8sc03837f日期:——A practical and readily scalable reaction sequence was developed for the straightforward synthesis of a new family of larger π-conjugated naphthopyrans by a Brønsted acid-catalyzed aromatic annulation of alkoxyallenes with inert naphthols. The cascade pathway involves allylation/cyclization/debenzyloxylation/isomerization/dehydration. The new class of solid state diphenylmethylene substituted naphthopyrans
表征谱图
-
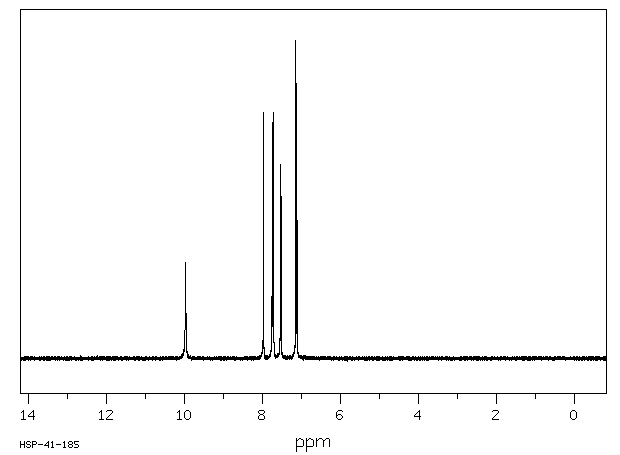
氢谱1HNMR
-
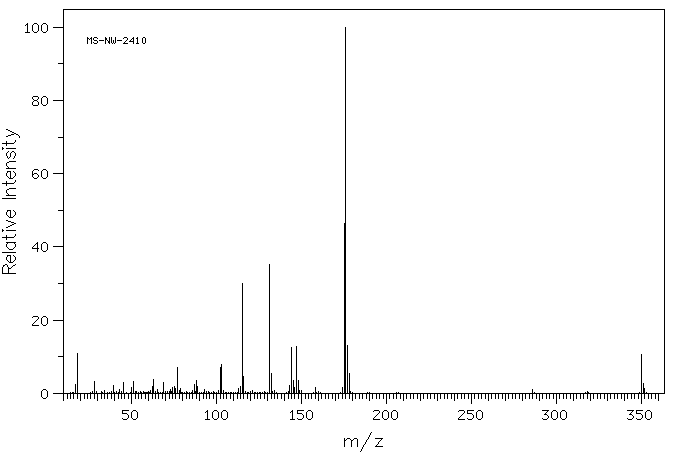
质谱MS
-
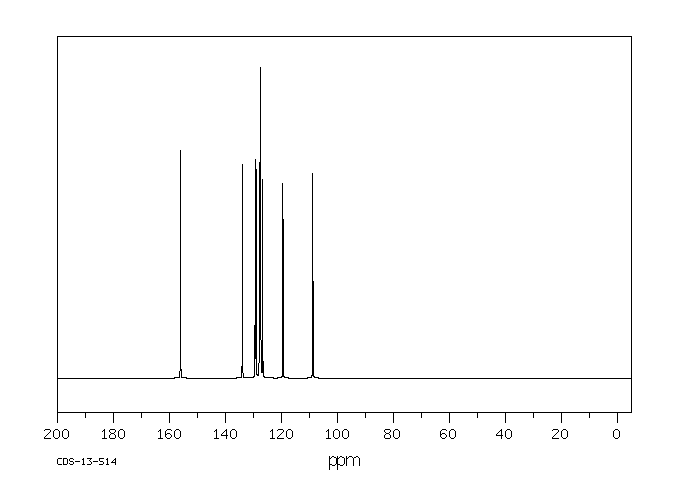
碳谱13CNMR
-
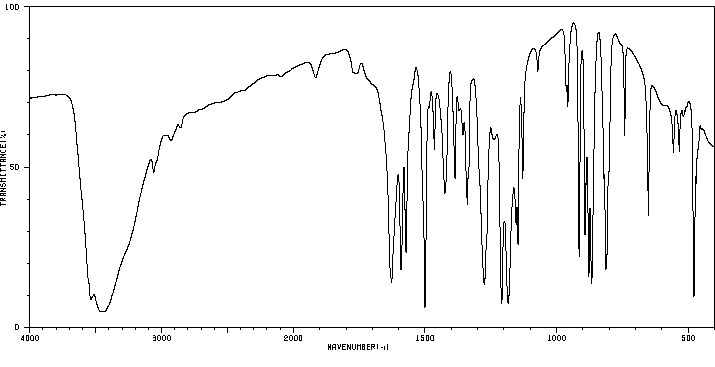
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮