1-adamantane carboxylic acid
中文名称
——
中文别名
——
英文名称
1-adamantane carboxylic acid
英文别名
adamantane-1-carboxylic acid;adamantine-1-carboxylic acid;1-adamantanecarboxylic acid;adamantane carboxylic acid
CAS
——
化学式
C11H16O2
mdl
——
分子量
180.247
InChiKey
JIMXXGFJRDUSRO-KJZNFTALSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.29
-
重原子数:13.0
-
可旋转键数:1.0
-
环数:4.0
-
sp3杂化的碳原子比例:0.91
-
拓扑面积:37.3
-
氢给体数:1.0
-
氢受体数:1.0
反应信息
-
作为反应物:描述:1-adamantane carboxylic acid 在 对甲苯二硫醚 、 10-(3,5-dimethoxyphenyl)-9-mesityl-1,3,6,8-tetramethoxyacridin-10-ium tetrafluoroborate 、 四丁基醋酸铵 作用下, 以 乙酸乙酯 为溶剂, 反应 12.0h, 以93%的产率得到金刚烷参考文献:名称:可见光光氧化还原催化脂肪酸的加氢脱羧和氘脱羧摘要:我们为烷烃和氘烷烃开发了一种高效的可见光光氧化还原催化的脂肪酸加氢脱羧和氘脱羧。高效转化的关键在于合适的甲氧基取代吖啶光催化剂(Mes-1,3,6,8-tetramethoxy-Acr-3”,5”-dimethoxy-Ph)的共催化和氢原子转移催化剂 4',4'-二甲基二苯二硫烷,可能通过促进活性烷基自由基猝灭以克服竞争性均偶联产物,烯烃通过歧化反应,裂解产物通过 C-C 键 β-断裂。DOI:10.1002/cjoc.202200143
-
作为产物:描述:adamantane carboxylic acid p-nitrophenyl ester 在 Pseudomonas aeruginosa D1F8 lipase 作用下, 以 乙腈 为溶剂, 反应 1.5h, 以15%的产率得到1-adamantane carboxylic acid参考文献:名称:扩大酶的底物范围:结合通过CASTing获得的突变。摘要:在先前的论文中,引入了组合活性位点饱和度测试(CAST)作为将酶定向进化为更广泛的底物接受度的有效策略。CASTing包括系统设计和围绕完整结合口袋的聚焦文库的筛选,但这只是进化过程的第一步,因为仅考虑了突变体的初始文库。在本研究中,通过组合从两个不同文库获得的突变变化,提出了一种用于进一步优化初始命中的简单方法。筛选组合的脂肪酶突变体针对六种众所周知的困难底物(大分子羧酸酯)的水解活性,并鉴定出显示出明显更高活性的改良突变体。还研究了在两种底物的水解动力学拆分中突变体的对映选择性,最佳的突变体-底物组合导致选择性因子为E = 49。最后,出于理论原因,研究了进化的突变体在简单的非支链羧酸酯(从乙酸酯到棕榈酸酯)的水解中的催化特性。DOI:10.1002/chem.200600459
文献信息
-
[EN] IMIDAZOTRIAZINONE COMPOUNDS<br/>[FR] COMPOSÉS D'IMIDAZOTRIAZINONE申请人:ENVIVO PHARMACEUTICALS INC公开号:WO2013142269A1公开(公告)日:2013-09-26The present invention provides imidazotriazinone compounds which are inhibitors of phosphodiesterase 9 and pharmaceutically acceptable salt thereof. The present invention further provides processes, pharmaceutical compositions, pharmaceutical preparations and pharmaceutical use of the compounds in the treatment of PDE9 associated diseases or disorders in mammals, including humans.
-
Synthesis of Cage Acylamino Derivatives in Nitric Acid Medium作者:Yu. N. Klimochkin、M. V. Leonova、E. A. Ivleva、A. I. Kazakova、M. S. ZaborskayaDOI:10.1134/s1070428021010012日期:2021.1Abstract An efficient procedure has been developed for the synthesis of secondary amides by the Ritter reaction of cage substrates with a wide series of nitriles in fuming nitric acid and its mixtures.摘要 已经开发出一种有效的方法,用于通过笼式底物在发烟硝酸及其混合物中与一系列腈进行Ritter反应来合成仲酰胺。
-
Synthesis of α-Amidoketones through the Cascade Reaction of Carboxylic Acids with Vinyl Azides under Catalyst-Free Conditions作者:Cai Gao、Qianting Zhou、Li Yang、Xinying Zhang、Xuesen FanDOI:10.1021/acs.joc.0c01871日期:2020.11.6An efficient synthesis of α-amidoketone derivatives through the cascade reactions of carboxylic acids with vinyl azides is presented. Compared with literature protocols, notable features of this new method include catalyst-free conditions, broad substrate scope, good tolerance of a wide range of functional groups, and high efficiency. In addition, the synthetic potential of this method as a tool for
-
General and selective <i>syn</i>-carboxylation-trifluoromethylation of terminal alkynes: application to the late-stage modification of dehydrocholic acid作者:Song-Lin Zhang、Chang Xiao、Hai-Xing Wan、Xiaoming ZhangDOI:10.1039/c9cc01173k日期:——alkynes by a Cu(III)–CF3 complex and a carboxylic acid regioselectively and with unusual syn-stereochemistry. This method is broadly applicable to various carboxylic acids and terminal alkynes, producing a range of biologically active trifluoromethyl enol carboxylic esters. The synthetic potential of this method is further demonstrated by the late-stage functionalization of a complex pharmaceutical compound
-
Gold Catalysis: No Steric Limitations in the Phenol Synthesis作者:A. Stephen K. Hashmi、Ralph Salathé、Wolfgang FreyDOI:10.1002/chem.200600533日期:2006.9.6Different dihydroisoindol-4-ols and 8-hydroxytetrahydroisoquinolines were prepared by the gold-catalyzed phenol synthesis. The reaction was investigated with several sterically demanding groups in the 5-position of the furan starting material. The influence of the reaction time and the catalyst on the yield was investigated.
表征谱图
-
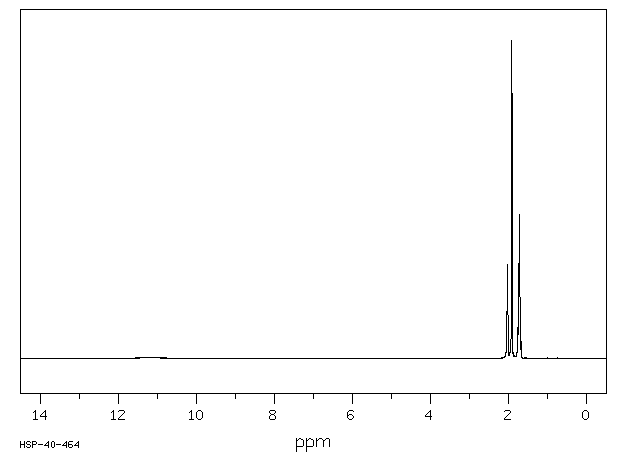
氢谱1HNMR
-
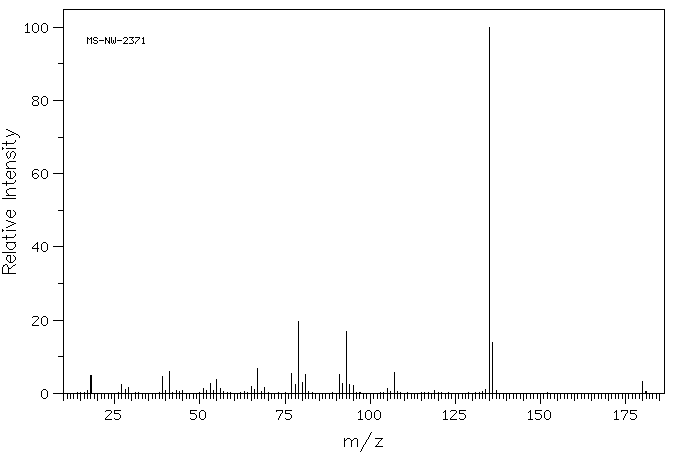
质谱MS
-
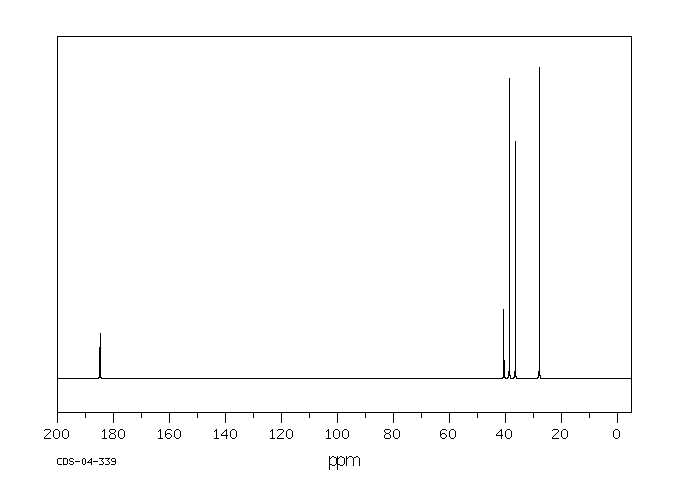
碳谱13CNMR
-
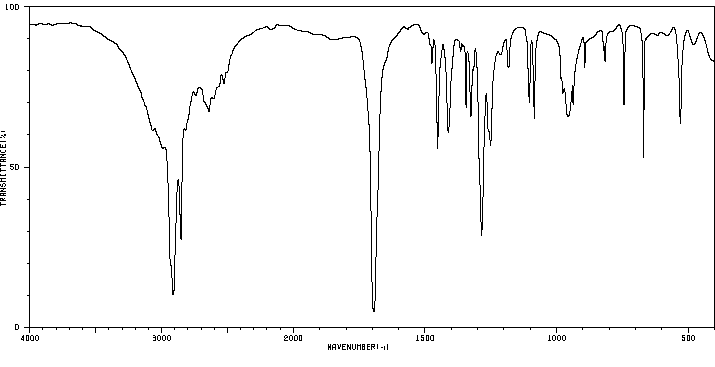
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸