(2-碘-5-甲氧基苯基)甲醇 | 139557-96-3
中文名称
(2-碘-5-甲氧基苯基)甲醇
中文别名
——
英文名称
(2-iodo-5-methoxyphenyl)methanol
英文别名
2-iodo-5-methoxybenzyl alcohol
CAS
139557-96-3
化学式
C8H9IO2
mdl
——
分子量
264.063
InChiKey
AYLPYLQTOJCHSH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64-65 °C(Solv: ethyl ether (60-29-7))
-
沸点:329.8±32.0 °C(Predicted)
-
密度:1.768±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-碘-5-甲氧基苯甲酸 2-iodo-5-methoxybenzoic acid 54413-93-3 C8H7IO3 278.046 2-碘-5-甲氧基苯(甲)醛 2-iodo-5-methoxybenzaldehyde 77287-58-2 C8H7IO2 262.047 间甲氧基苯甲醇 3-methoxybenzyl alcohol 6971-51-3 C8H10O2 138.166 2-溴-5-甲氧基苄醇 2-Bromo-5-methoxybenzyl alcohol 150192-39-5 C8H9BrO2 217.062 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-碘-5-甲氧基苯(甲)醛 2-iodo-5-methoxybenzaldehyde 77287-58-2 C8H7IO2 262.047 —— 2-(bromomethyl)-1-iodo-4-methoxybenzene 293732-24-8 C8H8BrIO 326.959 间甲氧基苯甲醇 3-methoxybenzyl alcohol 6971-51-3 C8H10O2 138.166 —— 3-(2-iodo-5-methoxyphenyl)propanoic acid 99254-50-9 C10H11IO3 306.1
反应信息
-
作为反应物:描述:参考文献:名称:Anionic Fries rearrangements of esters of ortho-iodobenzyl alcohols: rapid routes to oestrone methyl ether and its 9? epimer, and aryl naphthalide lignans摘要:通过锂-碘交换引发的快速、通用、低温邻碘苄酯重排反应,生成异苯并呋喃,随后通过分子间和分子内迪尔斯-阿尔德(IMDA)反应当场截获,生成多种碳环化合物,包括天然木脂素和甾体。DOI:10.1039/c39920000164
-
作为产物:描述:参考文献:名称:羰基化[2 + 2 + 1]环加成反应的进展:腈基作为π组分的利用摘要:新的技巧,旧的反应:在催化条件下处理催化量为[{RhCl(CO)dppp} 2 ](dppp = 1,3-‐双(二苯基膦基)丙烷的2-(1,2-丙二烯基)苯基乙腈衍生物CO气氛下生成苯并[ f ]氧基吲哚衍生物(参见方案)。该aza-Pauson-Khand型反应适用于脂肪族底物,从而导致形成azabicyclo [3.3.0] octadienone衍生物。DOI:10.1002/anie.201305729
文献信息
-
Enantioselective Allylation of Selected<i>ortho</i>-Substituted Benzaldehydes: A Comparative Study作者:Filip Hessler、Robert Betík、Aneta Kadlčíková、Roman Belle、Martin KotoraDOI:10.1002/ejoc.201403034日期:2014.11Lewis base catalysis proved to be more efficient, and the highest asymmetric induction for allylation of ortho-fluorobenzaldehyde reached 82 % ee, which is comparable to other used catalytic conditions. In cases of ortho-vinylbenzaldehyde, the Keck allylation provided the product in 88 % ee. An enantioenriched homoallylic alcohol was used as the starting material for the synthesis of a sertraline
-
Palladium-catalyzed intermolecular tandem cyclization reaction: a highly regioselective synthesis of functionalized 3H-spiro[isobenzofuran-1,3′-isochroman] scaffolds作者:Liang Wang、Xuehu Li、Hua Tao、Xiang Zhou、Xihong Lu、Wenyue Du、Tingting Jiang、Zhijun Xin、Jianping LiangDOI:10.1039/c6ob02802k日期:——functionalized 3H-spiro[isobenzofuran-1,3′-isochroman] scaffolds using a novel palladium-catalyzed tandem cyclization reaction is explored. During the reaction process, C–O, C–C and C–O bonds are sequentially formed in one pot via decarboxylative allenylpalladium formation, nucleophilic attack, arylpalladium addition and intramolecular nucleophilic attack.
-
Nickel‐Catalyzed Asymmetric Reductive 1,2‐Carboamination of Unactivated Alkenes作者:Jun He、Yuhang Xue、Bo Han、Chunzhu Zhang、You Wang、Shaolin ZhuDOI:10.1002/anie.201913743日期:2020.2.3Starting from diverse alkene-tethered aryl iodides and O-benzoyl-hydroxylamines, the enantioselective reductive cross-electrophilic 1,2-carboamination of unactivated alkenes was achieved using a chiral pyrox/nickel complex as the catalyst. This mild, modular, and practical protocol provides rapid access to a variety of β-chiral amines with an enantioenriched aryl-substituted quaternary carbon center
-
Copper‐Catalyzed Azide–Ynamide Cyclization to Generate α‐Imino Copper Carbenes: Divergent and Enantioselective Access to Polycyclic N‐Heterocycles作者:Xin Liu、Ze‐Shu Wang、Tong‐Yi Zhai、Chen Luo、Yi‐Ping Zhang、Yang‐Bo Chen、Chao Deng、Rai‐Shung Liu、Long‐Wu YeDOI:10.1002/anie.202007206日期:2020.10.5Here an efficient copper‐catalyzed cascade cyclization of azide‐ynamides via α‐imino copper carbene intermediates is reported, representing the first generation of α‐imino copper carbenes from alkynes. This protocol enables the practical and divergent synthesis of an array of polycyclic N‐heterocycles in generally good to excellent yields with broad substrate scope and excellent diastereoselectivities
-
Direct Intramolecular Aminoboration of Allenes作者:Chun-Hua Yang、Meng Han、Wenyan Li、Ningning Zhu、Zhenzhen Sun、Junjie Wang、Zhantao Yang、Yue-Ming LiDOI:10.1021/acs.orglett.0c01685日期:2020.7.2Direct intramolecular aminoboration of sulfonamidoallenes was realized using BCl3 as a boron source. The reactions benefited from the interaction between BCl3 and sulfonamides and provided a variety of borylvinyl heterocycles in good isolated yields. When chiral substrates were involved in the reactions, high stereoselectivity was observed, as can be ascertained by single-crystal X-ray diffraction
表征谱图
-
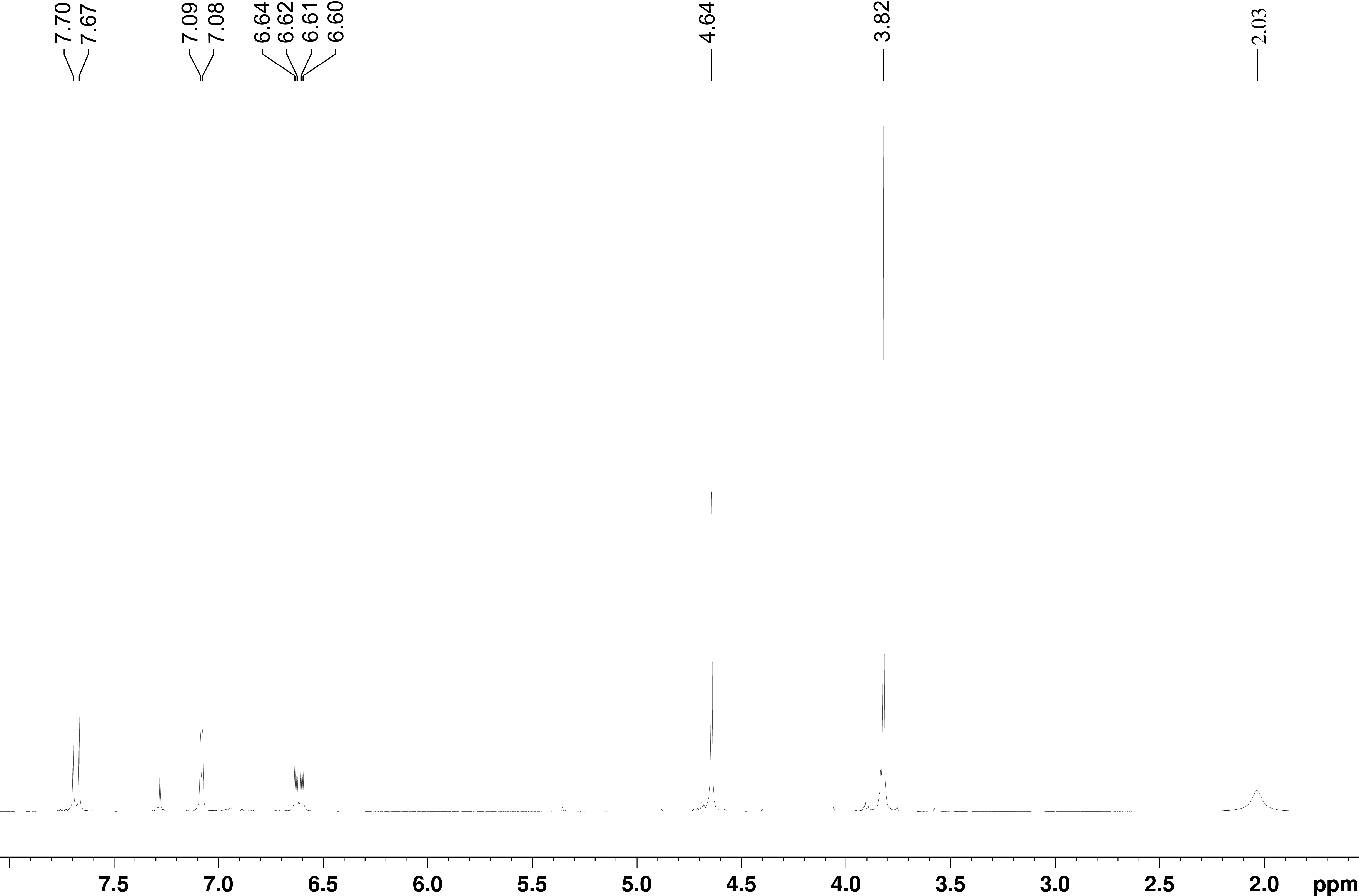
氢谱1HNMR
-
质谱MS
-
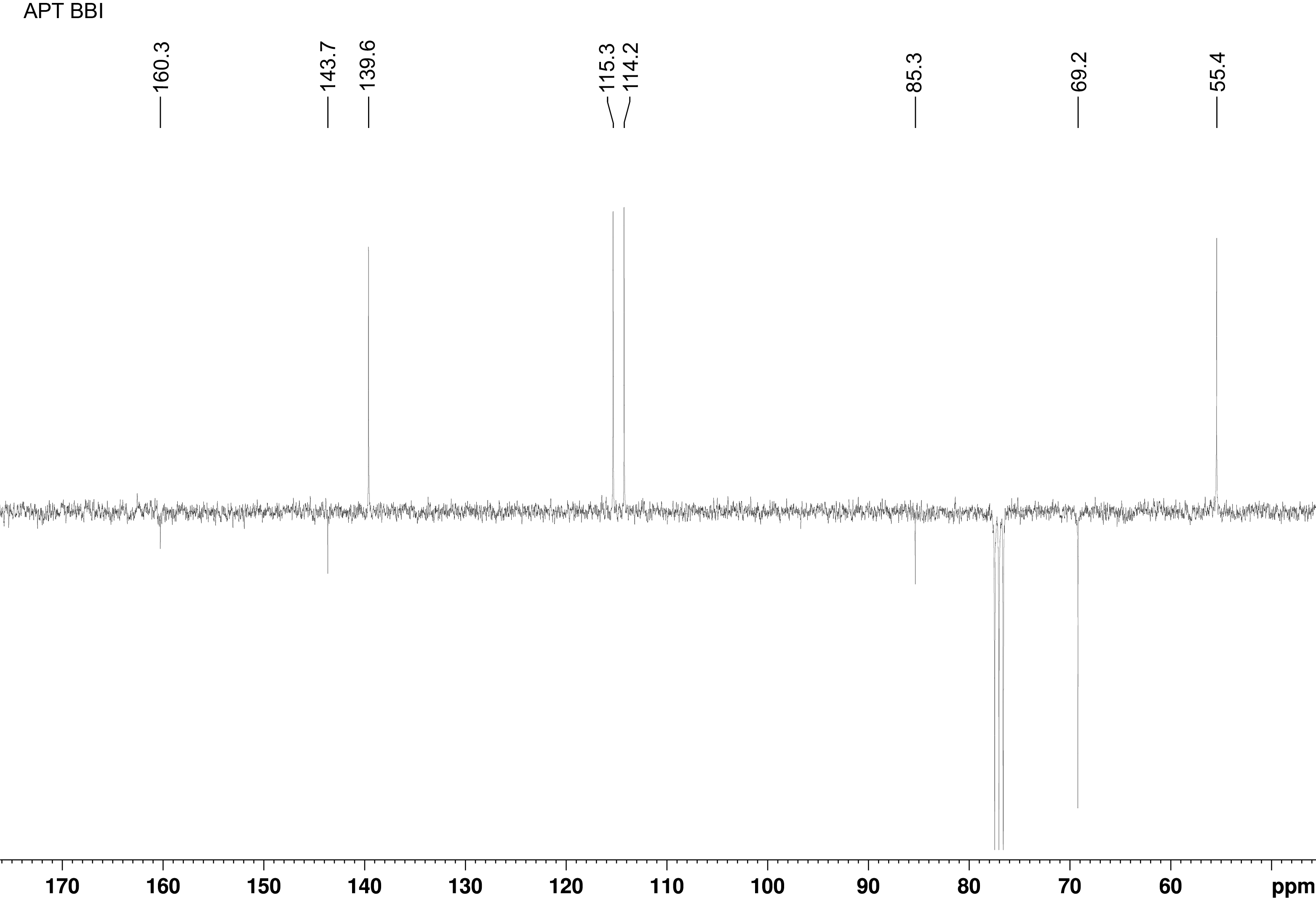
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫