1-(吡咯烷基羰基甲基)哌嗪 | 39890-45-4
中文名称
1-(吡咯烷基羰基甲基)哌嗪
中文别名
1-(吡咯烷羰基亚甲基)哌嗪;1-[(1-四氢吡咯羰基)甲基]哌嗪;马来酸桂哌齐特中间体;1-(1-哌嗪乙酰基)吡咯啶;1-(2-哌嗪-1-基乙酰)吡咯烷;1-吡咯甲酰哌嗪
英文名称
1-[(pyrrolidine-1-carbonyl)methyl]piperazine
英文别名
2-piperazin-1-yl-1-pyrrolidin-1-ylethanone;1-(Pyrrolidinocarbonylmethyl)piperazine
CAS
39890-45-4
化学式
C10H19N3O
mdl
MFCD00005968
分子量
197.28
InChiKey
KYBCXTTWIOZBNR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75 °C
-
沸点:356.2±27.0 °C(Predicted)
-
密度:1.092±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.9
-
拓扑面积:35.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
海关编码:2933990090
-
安全说明:S26,S36
-
储存条件:2-8℃
SDS
| Name: | 1-(Pyrrolidinocarbonylmethyl)piperazine 95+% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 39890-45-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 39890-45-4 | 1-(pyrrolidinocarbonylmethyl)piperazin | 95+ | 254-677-4 |
Risk Phrases:
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Appearance: yellow.
Not available.
Target Organs: None.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits +--------------------+-------------------+-------------------+-----------------+ | Chemical Name | ACGIH | NIOSH |OSHA - Final PELs| |--------------------|-------------------|-------------------|-----------------| | 1-(pyrrolidinocarbo|none listed |none listed |none listed | | nylmethyl)piperazin| | | | | e, 95+% | | | | +--------------------+-------------------+-------------------+-----------------+ OSHA Vacated PELs: 1-(pyrrolidinocarbonylmethyl)piperazine, 95+%: No OSHA Vacated PELs are listed for this chemical.
Personal Protective Equipment Eyes: Wear chemical goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant a respirator's use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Appearance: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 79.00 - 81.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
NFPA Rating: Not published.
Explosion Limits, Lower: Not available.
Upper: Not available.
Decomposition Temperature:
Solubility:
Specific Gravity/Density:
Molecular Formula: C10H19N3O
Molecular Weight: 197.28
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 39890-45-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(pyrrolidinocarbonylmethyl)piperazine, 95+% - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA.
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Chemical waste generators must determine whether a discarded chemical is classif as a hazardous waste.
US EPA guidelines for the classification determination are listed in 40 CFR Part Additionally, waste generators must consult state and local hazardous waste regu ensure complete and accurate classification.
RCRA P-Series: None listed.
RCRA U-Series: None listed.
Section 14 - TRANSPORT INFORMATION
CDG/CPL
Not classified as hazardous for supply.
Canadian TDG
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 39890-45-4: No information available.
United Kingdom Occupational Exposure Limits
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
WHMIS: Not available.
CAS# 39890-45-4 is not listed on Canada's Ingredient Disclosure List.
Exposure Limits
US FEDERAL
TSCA
CAS# 39890-45-4 is not listed on the TSCA inventory.
It is for research and development use only.
Health & Safety Reporting List
None of the chemicals are on the Health & Safety Reporting List.
Chemical Test Rules
None of the chemicals in this product are under a Chemical Test Rule.
Section 12b
None of the chemicals are listed under TSCA Section 12b.
TSCA Significant New Use Rule
None of the chemicals in this material have a SNUR under TSCA.
SARA
Section 302 (RQ)
None of the chemicals in this material have an RQ.
Section 302 (TPQ)
None of the chemicals in this product have a TPQ.
Section 313
No chemicals are reportable under Section 313.
Clean Air Act:
This material does not contain any hazardous air pollutants.
This material does not contain any Class 1 Ozone depletors.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(4-Methylpiperazin-1-yl)-1-pyrrolidin-1-ylethanone 120697-41-8 C11H21N3O 211.307 —— 2-(4-Acetylpiperazin-1-yl)-1-pyrrolidin-1-ylethanone 120697-45-2 C12H21N3O2 239.318 —— 4-oxo-4-[4-(2-oxo-2-pyrrolidin-1-ylethyl)piperazin-1-yl]butyric acid 318280-18-1 C14H23N3O4 297.354 —— 5-oxo-5-[4-(2-oxo-2-pyrrolidin-1-ylethyl)piperazin-1-yl]pentanoic acid 318280-33-0 C15H25N3O4 311.381 —— 3-methyl-5-oxo-5-[4-(2-oxo-2-pyrrolidin-1-ylethyl)piperazin-1-yl]pentanoic acid 318280-24-9 C16H27N3O4 325.408 —— 2-[4-[(4-Chlorophenyl)methyl]piperazin-1-yl]-1-pyrrolidin-1-ylethanone 119963-60-9 C17H24ClN3O 321.85
反应信息
-
作为反应物:描述:1-(吡咯烷基羰基甲基)哌嗪 在 盐酸 作用下, 以 乙醇 、 苯 为溶剂, 反应 14.0h, 生成 3-[4-(2-Oxo-2-pyrrolidin-1-ylethyl)piperazin-1-yl]-1-phenylpropan-1-one参考文献:名称:Hypolipidemic effects of α, β, and γ-alkylaminophenone analogs in rodents摘要:A number of N-substituted beta-alkylaminophenone derivatives including two alpha- and two gamma-alkylaminophenone analogs were synthesized and investigated for hypolipidemic activity in mice at 8 mg/kg/day ip. Most of these analogs were found to be significantly more active than lovastatin and clofibrate. N-Phenylpiperazinopropiophenone 16 was one of the best derivatives, lowering serum cholesterol levels 41% and serum triglyceride levels 48% after 16 days of drug administration in CF1 mice. In Sprague-Dawley rats, N-phenylpiperazinopropiophenone at 8 mg/kg/day orally also demonstrated more potent hypolipidemic activity than clofibrate, gemfibrozil, and lovastatin at their therapeutic dosage. It significantly reduced tissue cholesterol and triglyceride levels in the aorta wall tissue and lowered the cholesterol and triglyceride levels in chylomicron, very low density lipid (VLDL) and low density lipid (LDL) fractions, while it significantly elevated the cholesterol levels in high density lipid (HDL) fraction. This compound also proved to be active in lowering both cholesterol and triglyceride levels in hyperlipidemic mice and rats induced with atherogenic diet. In vitro liver acetyl coenzyme A (CoA) synthetase, 3-hydroxy-3-methyl glutaryl (HMG) CoA reductase, acyl CoA cholesterol acyl transferase (ACAT), sn-glycerol-3-phosphate acyltransferase, phosphatidylate phosphohydrolase, and hepatic lipoprotein lipase activities were significantly inhibited by N-phenylpiperazinopropiophenone from 25 to 100 mu M.DOI:10.1016/0223-5234(96)80365-5
-
作为产物:描述:哌嗪 生成 1-(吡咯烷基羰基甲基)哌嗪参考文献:名称:ITO, YASUO;KATO, XIDEHO;KOGAVA, NOBUO;KURADA, EHJ;YAMAGISI, TEHRKATO摘要:DOI:
文献信息
-
Compounds and uses thereof for decreasing activity of hormone-sensitive lipase申请人:——公开号:US20030166644A1公开(公告)日:2003-09-04Use of compounds to inhibit hormone-sensitive lipase, pharmaceutical compositions comprising the compounds, methods of treatment employing these compounds and compositions, and novel compounds. The present compounds are inhibitors of hormone-sensitive lipase and may be useful in the treatment and/or prevention of medical disorders where a decreased activity of hormone-sensitive lipase is desirable.
-
[EN] 6H-THIENO`2, 3-B!PYRROLE DERIVATIVES AS ANTAGONISTS OF GONADOTROPIN RELEASING HORMONE (GNRH)<br/>[FR] DERIVES DE 6H-THIENO`2,3-B!PYRROLE EN TANT QU'ANTAGONISTES DE LA GONADOLIBERINE (GNRH)申请人:ASTRAZENECA AB公开号:WO2004018480A1公开(公告)日:2004-03-04The invention relates to a group of novel thieno-pyrrole compounds of Formula (I): wherein: R1, R2, R3, R4 and R5 are as defined in the specification, which are useful as gonadotrophin releasing hormone antagonists. The invention also relates to pharmaceutical formulations of said compounds, methods of treatment using said compounds and to processes for the preparation of said compounds.
-
Parallel Solution-Phase Synthesis and General Biological Activity of a Uridine Antibiotic Analog Library作者:Omar Moukha-chafiq、Robert C. ReynoldsDOI:10.1021/co4001452日期:2014.5.12coupling of the amino terminus of d-phenylalanine methyl ester to the free 5′-carboxylic acid moiety of 33 followed by sodium hydroxide treatment led to carboxylic acid analog 77. Hydrolysis of this material gave analog 78. The intermediate 77 served as the precursor for the preparation of novel dipeptidyl uridine analogs 79–99 through peptide coupling reactions to diverse amine reactants. None of the described在 NIH 路线图计划的中试规模库 (PSL) 计划下,以溶液相方式从胺2和羧酸33和77合成了一个包含 94 种尿苷抗生素类似物的小型库。多样醛,磺酰氯,和羧酸反应物套缩合,2,酸介导的水解导致后,向目标化合物3 - 32以良好的收率和高的纯度。同样,用不同的胺和磺胺处理33得到34 – 75。d氨基末端的偶联-苯丙氨酸甲酯到33的游离 5'-羧酸部分,然后用氢氧化钠处理产生羧酸类似物77。该材料的水解得到类似物78。中间77担任该前体为二肽基新颖尿苷类似物的制备79 - 99通过肽偶联到不同的胺反应剂反应。所描述的化合物均未显示出显着的抗癌或抗疟活性。通过 NIH MLPCN 计划提供和报告的初级筛选中的酶和受体,许多样品表现出各种有希望的抑制性、激动剂、拮抗剂或激活剂特性。
-
[EN] PROTEIN KINASE INHIBITORS AND USE THEREOF<br/>[FR] INHIBITEURS DE PROTÉINE KINASE ET LEUR UTILISATION申请人:MERCK SERONO SA公开号:WO2009108670A1公开(公告)日:2009-09-03Disclosed are benzonaphthyridinyl derivative compounds and analogs thereof, pharmaceutical compositions comprising such compounds and processes for preparing the same. The compounds are useful in the treatment of diseases amenable to kinase signal transduction inhibition, regulation or modulation.
-
SUBSTITUTED PYRIDINYL-PYRIMIDINES AND THEIR USE AS MEDICAMENTS申请人:Dahmann Georg公开号:US20130023502A1公开(公告)日:2013-01-24The invention relates to new substituted pyridinyl-pyrimidines of formula 1 wherein ring A is a five-membered saturated or unsaturated carbocyclic ring which optionally comprises one, two or three heteroatoms each independently from each other selected from the group N, S and O, wherein R 1 , R 2 , R 4 , R 3 , R 5 and R 6 are defined as in claim 1 and wherein ring A is further optionally substituted by one or two further substituents and the pharmaceutically acceptable salts, diastereomers, enantiomers, racemates, hydrates and solvates of the aforementioned compounds.
表征谱图
-
氢谱1HNMR
-
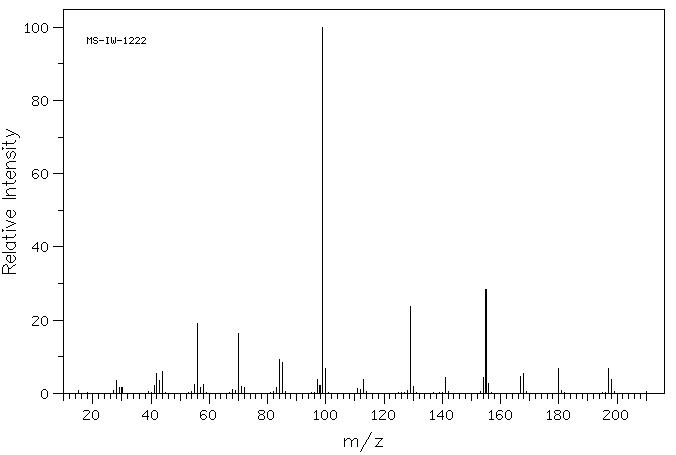
质谱MS
-
碳谱13CNMR
-
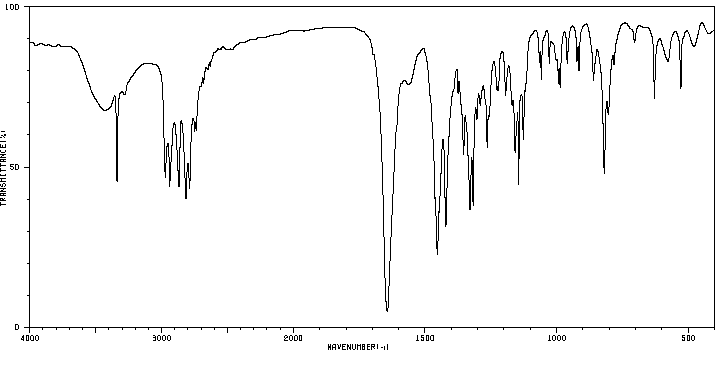
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2S)-4-[7-(8-氯-1-萘)-5,6,7,8-四氢-2-[[((2S)-1-甲基-2-吡咯烷基]甲氧基]吡啶基[3,4-d]嘧啶-4-基]-1-(2-氟-1-氧代-2-丙烯-1-基)-2-哌Chemicalbook嗪乙腈;2-((S)-4-(7-(8-氯萘-1-基)-2-((((S)-1-
齐拉西酮相关物质C
齐拉西酮杂质E
齐拉西酮开环物,氨基酸杂质
齐拉西酮亚砜
齐拉西酮 盐酸盐 一水合物
齐拉西酮
鲸蜡硬脂醇
鲁拉西酮杂质3
鲁拉西酮杂质23
鲁拉西酮杂质14
鲁拉西酮杂质11
鲁拉西酮
鲁拉西杂质E
鲁拉西杂质1
高分子量聚合三嗪类无卤阻燃剂
驱虫灵D
马福拉嗪
马来酸阿伐曲泊帕
顺式-1-乙酰基-2,6-二甲基-4-亚硝基-哌嗪
雷诺嗪双(N-氧化物)
陶扎色替
阿达色林
阿莫西林二氧代哌嗪
阿立哌唑羟基丁基杂质
阿立哌唑杂质26
阿立哌唑USP相关物质H
阿立哌唑N4-氧化物
阿立哌唑N,N-二氧化物
阿立哌唑EP杂质D
阿立哌唑-d8
阿立哌唑
阿泊替尼
阿替韦啶
阿拉诺丁
阿扎哌醇
阿得巴司
阿尔哌汀
阿伐曲泊帕杂质
间羟基苯基哌嗪
钾 1-甲基-4-三氟硼酸三甲基哌嗪
钠4-(4-乙酰基-1-哌嗪基)苯酚
酮齐拉西酮
酮酮唑油酸酯
酚酞单磷酸酯二-(2-氨基-2-甲基-1,3-丙二醇)盐
选择性氟试剂II
达鲁舍替
达哌唑
赫普索
赤霉素A7甲酯