1-氯-4-苯基硫基磺酰基-苯 | 1142-97-8
中文名称
1-氯-4-苯基硫基磺酰基-苯
中文别名
4-氯苯硫代磺酸-S-苯基酯
英文名称
phenyl 4-chlorobenzenethiosulfonate
英文别名
S-phenyl 4-chlorobenzenesulfonothioate;4-Chlor-benzolthiosulfonsaeure-S-phenylester;Benzenesulfonothioic acid, 4-chloro-, S-phenyl ester;1-chloro-4-phenylsulfanylsulfonylbenzene
CAS
1142-97-8
化学式
C12H9ClO2S2
mdl
——
分子量
284.787
InChiKey
OZBQHMNNLSUKDP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-86.8°C
-
密度:0.8341
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:67.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2930909090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Klivenyi et al., Acta Chimica Academiae Scientiarum Hungaricae, 1955, vol. 6, p. 373,378摘要:DOI:
-
作为产物:描述:参考文献:名称:1-烯丙基-2-乙炔基苯并咪唑与硫代磺酸盐的可见光诱导自由基级联反应组装硫代磺酰化吡咯并[1,2-a]苯并咪唑摘要:开发了可见光诱导的 1-烯丙基-2-乙炔基苯并咪唑与硫代磺酸盐的自由基多米诺反应,以中等至良好的产率生成硫代磺酰化吡咯并[1,2- a ]苯并咪唑。该反应在不含过渡金属的条件下进行,具有良好的官能团耐受性和高区域选择性。通过光催化促进的能量转移途径激活了涉及硫代磺酸盐的可能途径。DOI:10.1021/acs.joc.1c02082
文献信息
-
Copper-Catalyzed TBHP-Mediated Radical Cross-Coupling Reaction of Sulfonylhydrazides with Thiols Leading to Thiosulfonates作者:Guo-Yu Zhang、Shuai-Shuai Lv、Adedamola Shoberu、Jian-Ping ZouDOI:10.1021/acs.joc.7b01121日期:2017.9.15A tert-butyl hydroperoxide (TBHP)-mediated coupling of sulfonylhydrazides with thiols catalyzed by CuBr2 to afford thiosulfonates via a radical process is described.
-
H<sub>2</sub>O<sub>2</sub>-mediated metal-free protocol towards unsymmetrical thiosulfonates from sulfonyl hydrazides and disulfides in PEG-400作者:Zhihong Peng、Xiao Zheng、Yingjun Zhang、Delie An、Wanrong DongDOI:10.1039/c8gc00381e日期:——A green and practical protocol between sulfonyl hydrazides and disulfides is herein reported for the synthesis of unsymmetrical thiosulfonates with the assistance of H2O2 in PEG-400, releasing N2 and H2O as the byproducts. The efficient and compatible process was considered to take place in the absence of metallic catalysts through a radical mechanism as determined by EPR analysis.
-
Visible-light-promoted synthesis of secondary and tertiary thiocarbamates from thiosulfonates and <i>N</i>-substituted formamides作者:Wen-Zhu Bi、Wen-Jie Zhang、Zi-Jie Li、Yuan-Hao He、Su-Xiang Feng、Yang Geng、Xiao-Lan Chen、Ling-Bo QuDOI:10.1039/d1ob01592c日期:——A general visible-light-promoted metal-free synthesis of secondary and tertiary thiocarbamates starting from thiosulfonates and N-substituted formamides is developed. By employing rhodamine B as a photocatalyst and tert-butyl hydroperoxide (TBHP) as an oxidant, a wide scope of thiocarbamates can be obtained through direct thiolation of acyl C–H bonds under irradiation of blue light at room temperature
-
Metal-Free Chemoselective Reaction of Sulfoxonium Ylides and Thiosulfonates: Diverse Synthesis of 1,4-Diketones, Aryl Sulfursulfoxonium Ylides, and β-Keto Thiosulfones Derivatives作者:Fei Wang、Bo-Xi Liu、Weidong Rao、Shun-Yi WangDOI:10.1021/acs.orglett.0c02370日期:2020.8.21A diverse chemoselective insertion reaction of sulfoxonium ylides and thiosulfonates under transition-metal-free conditions is developed, which successfully affords 1,4-diketone compounds, arylthiosulfoxide-ylides, and β-keto thiosulfones, respectively. The nucleophilic addition of two molecular sulfoxonium ylides to construct sulfone-substituted 1,4-dione compounds is the highlight of this work.
-
NBS‐Promoted Sulfenylation of Sulfinates with Disulfides Leading to Unsymmetrical or Symmetrical Thiosulfonates作者:Gaigai Liang、Miaochang Liu、Jiuxi Chen、Jinchang Ding、Wenxia Gao、Huayue WuDOI:10.1002/cjoc.201200028日期:2012.7practical method to access unsymmetrical and symmetrical thiosulfonates in moderate to excellent yields has been developed through NBS‐promoted sulfenylation of sulfinates with disulfides. The present process enables the use of two RS in RSSR and shows broad functional group tolerance, which represents an atom‐economical and practical procedure for the synthesis of thiosulfonates. A plausible mechanism
表征谱图
-
氢谱1HNMR
-
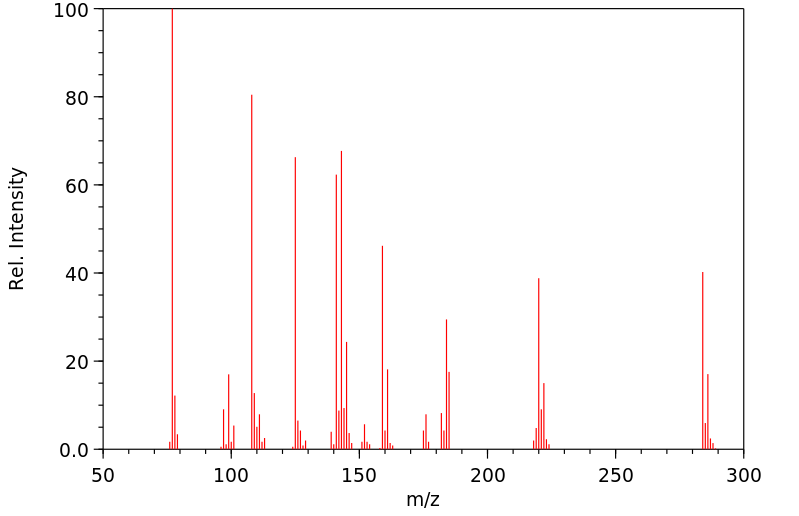
质谱MS
-
碳谱13CNMR
-
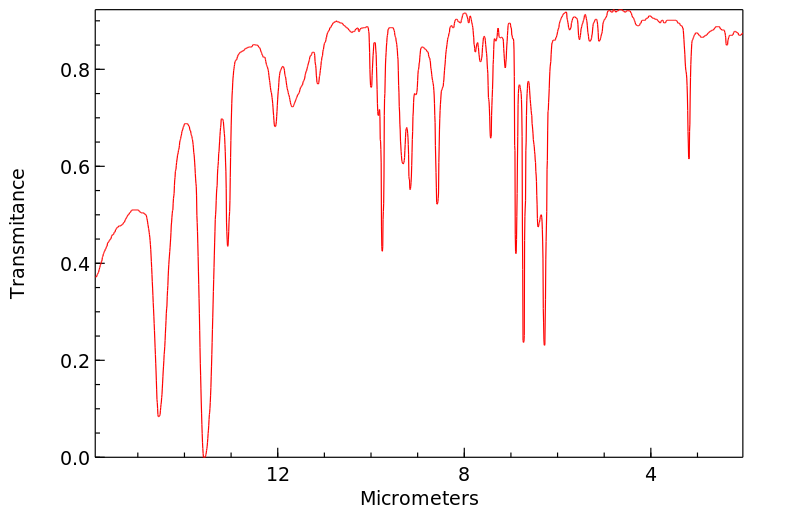
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫