1-环戊烯苯甲烷 | 15507-35-4
中文名称
1-环戊烯苯甲烷
中文别名
——
英文名称
1-benzylcyclopentene
英文别名
1-Benzylcyclopenten;1-Benzyl-cyclopenten-(1);(cyclopent-1-en-1-ylmethyl)benzene;1-Cyclopentenylphenylmethane;cyclopenten-1-ylmethylbenzene
CAS
15507-35-4
化学式
C12H14
mdl
MFCD00060808
分子量
158.243
InChiKey
GSXCQGUOLCBELU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:113-117 °C(Press: 18 Torr)
-
密度:0.958 g/cm3(Temp: 22 °C)
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:12
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:参考文献:名称:区域和对映选择性拜耳-维利格氧化:外消旋2-取代的环戊烷酮的动力学拆分。摘要:通过高度区域和对映选择性的Baeyer-Villiger氧化,成功开发了外消旋2-取代的环戊酮的动力学拆分。该反应可提供高达98%ee和> 19/1区域选择性的正常6-取代的δ-内酯。同时,未反应的酮以优异的ee值(高达98%)被回收。它代表通过非酶不对称BV氧化动力学拆分外消旋2取代的环戊酮的最佳结果。DOI:10.1021/ol501737a
-
作为产物:参考文献:名称:Cyclopentylphenylcarbinyl cation system摘要:DOI:10.1021/ja01035a019
文献信息
-
<i>B</i>-Alkylcatecholborane-Mediated Tandem Radical Conjugated Addition−Aldol Cyclization作者:Alice Beauseigneur、Cecilia Ericsson、Philippe Renaud、Kurt SchenkDOI:10.1021/ol9014943日期:2009.8.20A one-pot procedure involving radical conjugate addition of B-alkylcatecholboranes to enones followed by intramolecular aldol reaction is reported. Application to the stereoselective synthesis of monocyclic and bicyclic products with up to four contiguous stereogenic centers is presented.
-
Stereoselective Synthesis of δ-Lactones from 5-Oxoalkanals via One-Pot Sequential Acetalization, Tishchenko Reaction, and Lactonization by Cooperative Catalysis of Samarium Ion and Mercaptan作者:Jue-Liang Hsu、Jim-Min FangDOI:10.1021/jo016058t日期:2001.12.1sequence of acetalization, Tishchenko reaction and lactonization. The deliberative use of mercaptan, by comparison with alcohol, is advantageous to facilitate the catalytic cycle. The reaction mechanism and stereochemistry are proposed and supported by some experimental evidence. Such samarium ion/mercaptan cocatalyzed reactions show the feature of remote control, which is applicable to the asymmetric synthesis
-
Grubbs Metathesis Enabled by a Light‐Driven <i>gem</i> ‐Hydrogenation of Internal Alkynes作者:Tobias Biberger、Raphael J. Zachmann、Alois FürstnerDOI:10.1002/anie.202007030日期:2020.10.12complexes instigate a light‐driven gem‐hydrogenation of internal alkynes with concomitant formation of discrete Grubbs‐type ruthenium carbene species. This unorthodox reactivity mode is harnessed in the form of a “hydrogenative metathesis” reaction, which converts an enyne substrate into a cyclic alkene. The intervention of ruthenium carbenes formed in the actual gem‐hydrogenation step was proven by the
-
Alder-ene reaction of aryne with olefins作者:Zhao Chen、Jinhua Liang、Jun Yin、Guang-Ao Yu、Sheng Hua LiuDOI:10.1016/j.tetlet.2013.08.049日期:2013.10A novel intermolecular Alder-ene reaction based on aryne and olefins was developed. We performed this transformation under mild conditions such as at room temperature, and this reaction displayed high selectivity and good yields only in the presence of CsF. Hence, the intermolecular Alder-ene reaction of aryne with olefins provides an effective route to synthesize derivatives of olefins.
-
Convenient ‘one-flask’ synthesis of olefins. Reaction of α-chloroketones with Grignard reagents and lithium作者:José Barluenga、Miguel Yus、Pablo BernadDOI:10.1039/c3978000847a日期:——Olefins and diolefins with the double bonds in predetermined positions are prepared in a one-step process in moderate to good yields by the reaction of α-chloroketones with Grignard reagents and then with lithium.
表征谱图
-
氢谱1HNMR
-
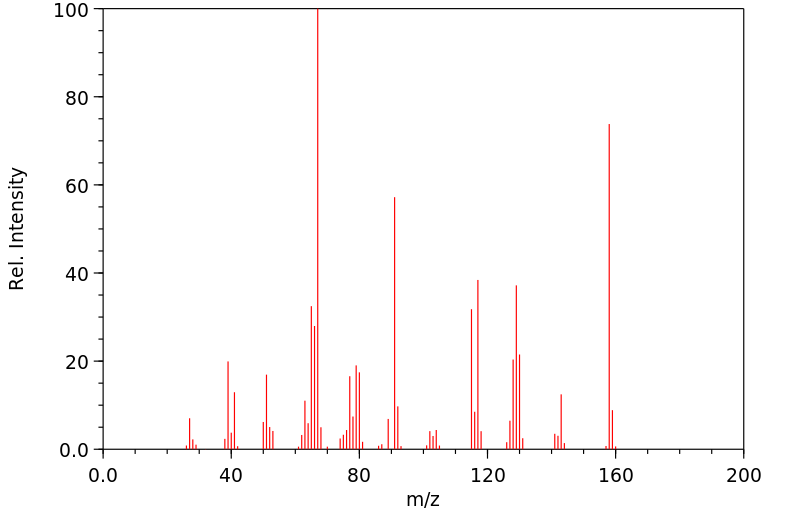
质谱MS
-
碳谱13CNMR
-
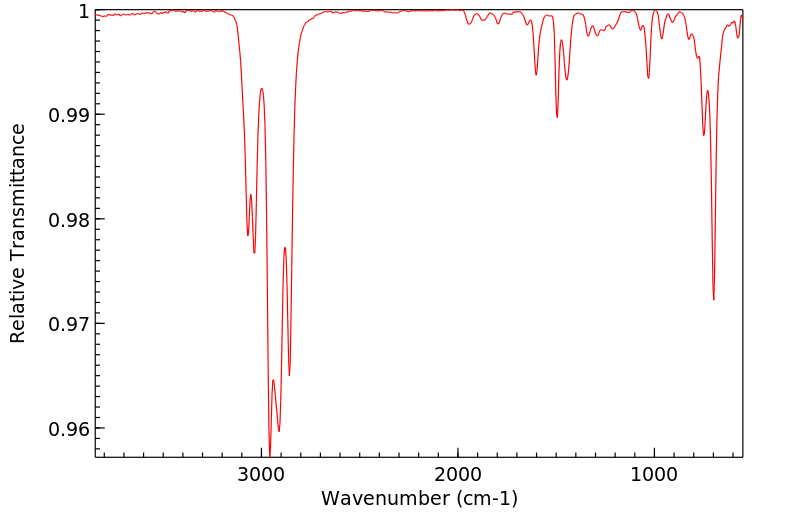
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫