1-苯基-1-壬炔 | 57718-18-0
中文名称
1-苯基-1-壬炔
中文别名
1-庚基-2-苯基乙炔;苯壬炔;1-庚-2-苯基乙炔
英文名称
non-1-yn-1-ylbenzene
英文别名
1-phenyl-1-nonyne;non-1-ynylbenzene
CAS
57718-18-0
化学式
C15H20
mdl
MFCD00048944
分子量
200.324
InChiKey
ZRQWNFWWPPYNFA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:295.2±9.0 °C(Predicted)
-
密度:0.81
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:15
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.466
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
-
储存条件:保持贮藏器密封,并将其存放在阴凉、干燥处。确保工作间有良好的通风或排气装置。
SDS
1-苯基-1-壬炔 修改号码:5
模块 1. 化学品
产品名称: 1-Phenyl-1-nonyne
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-苯基-1-壬炔
百分比: >98.0%(GC)
CAS编码: 57718-18-0
俗名: 1-Heptyl-2-phenylacetylene
分子式: C15H20
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
1-苯基-1-壬炔 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.89
溶解度:
[水] 无资料
[其他溶剂] 无资料
1-苯基-1-壬炔 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1-苯基-1-壬炔 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1-Phenyl-1-nonyne
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-苯基-1-壬炔
百分比: >98.0%(GC)
CAS编码: 57718-18-0
俗名: 1-Heptyl-2-phenylacetylene
分子式: C15H20
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
1-苯基-1-壬炔 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.89
溶解度:
[水] 无资料
[其他溶剂] 无资料
1-苯基-1-壬炔 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1-苯基-1-壬炔 修改号码:5
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:有机化学中受阻的有机硼基团。23.二甲磺隆稳定的碳负离子与芳族酮和醛的相互作用,生成烯烃。摘要:Dimesitylboron稳定的碳负离子与二芳基酮反应,在温和的条件下以良好的收率得到相应的烯烃。与芳族醛的反应更复杂,但是在所有情况下,通过用氯三甲基硅烷捕获中间体,然后用碳酸氢钠水溶液处理,可以以高收率获得E-烯烃。HF / CH 3 CN。用三氟乙酸酐处理相同的中间体主要得到Z-烯烃。考虑了这些重要过程的设计和机制。DOI:10.1016/s0040-4020(01)87981-3
-
作为产物:参考文献:名称:A Novel Transformation of Esters to Alkynes with 1-Substituted Benzotriazoles摘要:Reactions of lithio benzotriazol-1-yl derivatives 2, 11, and 25 with aromatic and aliphatic esters 3, 12, and 26 gave alpha-(benzotriazol-1-yl) ketones 4, 13, and 27, respectively, in high yields. Alternatively, alpha-(benzotriazol-1-yl) ketones 22 can be accessed by the reaction of alpha-(benzotriazol-1-yl) esters 20 with Grignard reagents. Condensation of 4, 13, 22, and 27 with (p-toluenesulfonyl)hydrazine provided p-tosylhydrazones 5, 14, 21, and 28. Treatment of hydrazones 5, 21, and 28 with n-butyllithium in diethyl ether resulted in the elimination of the tosyl group, dinitrogen, and benzotriazolyl group to afford the corresponding acetylenes 9, 23, and 29 in good yields. When alpha-(benzotriazol-1-yl) 1-alpha-phenoxy hydrazones 14 were treated with methyllithium, n-butyllithium, or phenyllithium, alkynes 18 were obtained, in which phenoxy groups were replaced by the lithium reagents.DOI:10.1021/jo962291t
文献信息
-
Mild and Phosphine-Free Iron-Catalyzed Cross-Coupling of Nonactivated Secondary Alkyl Halides with Alkynyl Grignard Reagents作者:Chi Wai Cheung、Peng Ren、Xile HuDOI:10.1021/ol501087m日期:2014.5.2cross-coupling of nonactivated secondary alkyl bromides and iodides with alkynyl Grignard reagents at room temperature has been developed. A wide range of secondary alkyl halides and terminal alkynes are tolerated to afford the substituted alkynes in good yields. A slight modification of the reaction protocol also allows for cross-coupling with a variety of primary alkyl halides.
-
Water as a Hydrogenating Agent: Stereodivergent Pd-Catalyzed Semihydrogenation of Alkynes作者:Chuan-Qi Zhao、Yue-Gang Chen、Hui Qiu、Lei Wei、Ping Fang、Tian-Sheng MeiDOI:10.1021/acs.orglett.9b00148日期:2019.3.1Palladium-catalyzed transfer semihydrogenation of alkynes using H2O as the hydrogen source and Mn as the reducing reagent is developed, affording cis- and trans-alkenes selectively under mild conditions. In addition, this method provides an efficient way to access various cis-1,2-dideuterioalkenes and trans-1,2-dideuterioalkenes by using D2O instead of H2O.
-
In(III)-Catalyzed Direct Regioselective Syntheses of 1-Naphthaldehyde Derivatives <i>via</i> a Hidden Aldehyde 1,3-Translocation and Disjointed CO<sub>2</sub> Extrusion作者:Sabera Sultana、Gisela A. González-Montiel、Samjhana Pradhan、Hari Datta Khanal、Sagar D. Nale、Paul Ha-Yeon Cheong、Yong Rok LeeDOI:10.1021/acscatal.1c00629日期:2021.6.4A highly regioselective annulation of 3-formylchromones with alkynes in the presence of indium triflate leads to the direct construction of diverse 1-naphthaldehyde derivatives. This one-pot de novo protocol features an unusual aldehyde 1,3-translocation and disjointed CO2 extrusion. This methodology is effective for the construction of structurally diverse 1-naphthaldehydes bearing chiral moieties
-
Copper-Catalyzed Intermolecular Alkynylation and Allylation of Unactivated C(sp<sup>3</sup>)–H Bonds via Hydrogen Atom Transfer作者:Lei Liang、Ge Guo、Chen Li、Song-Lin Wang、Yue-Hui Wang、Hai-Ming Guo、Hong-Ying NiuDOI:10.1021/acs.orglett.1c03298日期:2021.11.5We describe Cu-catalyzed intermolecular alkynylation and allylation of unactivated C(sp3)–H bonds with singly occupied molecular orbital-philes (SOMO-philes) via hydrogen atom transfer (HAT). Employing N-fluoro-sulfonamide as a HAT reagent, a set of substituted alkene and alkyne compounds were synthesized in high yields with good regioselectivity and functional-group compatibility. Late-stage functionalization
-
Photoinduced Nickel-Catalyzed Chemo- and Regioselective Hydroalkylation of Internal Alkynes with Ether and Amide α-Hetero C(sp<sup>3</sup>)–H Bonds作者:Hong-Ping Deng、Xuan-Zi Fan、Zhi-Hui Chen、Qing-Hua Xu、Jie WuDOI:10.1021/jacs.7b08158日期:2017.9.27with unfunctionalized ethers and amides was achieved in an atom-efficient and additive-free manner through the synergistic combination of photoredox and nickel catalysis. The protocol was effective with a wide range of internal alkynes, providing products in a highly selective fashion. Notably, the observed regioselectivity is complementary to conventional radical addition processes. Mechanistic investigations
表征谱图
-
氢谱1HNMR
-
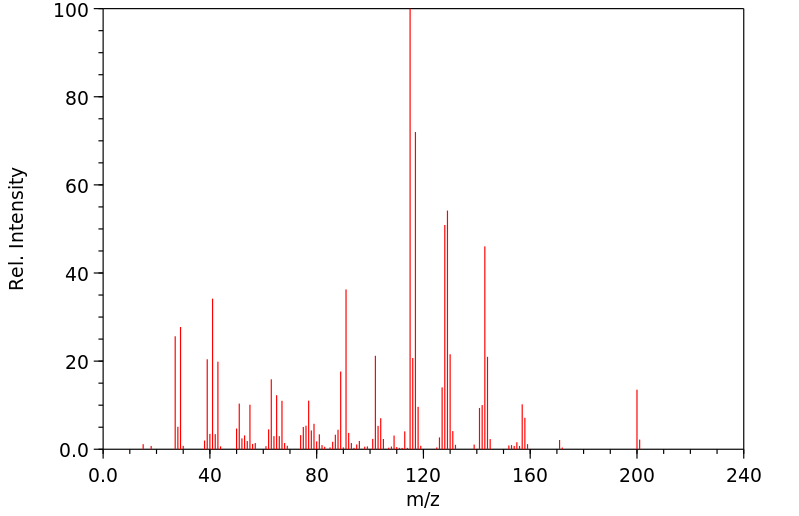
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫