氯乙醇磷酸酯 | 115-96-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-51 °C
-
沸点:192 °C/10 mmHg (lit.)
-
密度:1.39 g/mL at 25 °C (lit.)
-
闪点:450 °F
-
溶解度:H2O:可溶7.82g/L
-
LogP:1.7 at 20℃
-
物理描述:Tris(2-chloroethyl) phosphate is an odorless clear liquid. Neutral pH. (NTP, 1992)
-
颜色/状态:Clear, transparent liquid
-
气味:Low odor
-
蒸汽密度:Relative vapor density (air = 1): 9.8
-
蒸汽压力:6.13X10-2 mm Hg at 25 °C
-
稳定性/保质期:
Stable under recommended storage conditions.
-
自燃温度:480 °C
-
分解:Rapid decomposition occurs above 220 °C.
-
粘度:45 cP at 20 °C
-
折光率:Index of refraction: 1.4721 at 20 °C/D
-
保留指数:1740;1740;1740;1747.2;295.96
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:14
-
可旋转键数:9
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
危险等级:9
-
危险品标志:Xn
-
安全说明:S36/37,S61
-
危险类别码:R22,R51/53,R40
-
WGK Germany:3
-
海关编码:2919900090
-
危险品运输编号:UN 2810 6.1/PG 3
-
RTECS号:KK2450000
-
包装等级:Z01
SDS
制备方法与用途
磷酸三(2-氯乙基)酯(TCEP)是一种典型的有机磷系阻燃剂。它是一种微带奶油味的浅黄色油状液体,微溶于水。在聚合物中添加TCEP后,除了具有自熄性能外,还兼有防潮、防紫外线和抗静电等特点,并广泛用于纤维素、环氧树脂、酚醛树脂等材料以及纺织品、PVC材料和玩具等生活用品当中。
合成方法向系统中加入165 mL电解质、1.5 M氯化氢在乙烯氯丙烷中的溶液,以及0.5 M水。随后加入3.6 g称量过的红磷样品,并打开循环泵和冷却水。通入3 A的电流后,逐渐添加氯化氢和水在乙烯氯丙烷中的混合物,以确保电解过程中电解液中氯化氢和水分保持恒定浓度。电解完成后,过滤电解溶液去除磷。对获得的残留物进行称重,并根据差值确定溶解磷的重量。
接下来,在30至100°C和10-15毫米汞柱的压力下,通过喷水泵从滤液中用HCl和H2O蒸馏2.6 g乙烯氯丙烷,以获取被外加剂污染的原油残留物。随后在分液漏斗中使用20 mL 10% NaOH溶液洗涤,去除酸性添加剂;用水洗涤三次(每次30 mL),直至pH值中性,从而净化产品。最后,在真空条件下逐步升温至160°C和1.5 mmHg的条件下分离水残留物及挥发性外加剂,以获得磷酸三(2-氯乙基)酯。
化学性质TCEP为浅黄色油状液体,微带奶油味。它能与乙醇、丙酮、氯仿、四氯化碳等有机溶剂相溶,稍溶于水。
用途TCEP广泛用于聚氨酯泡沫阻燃以及聚氯乙烯阻燃增塑等。作为一种高效的阻燃剂,TCEP可以作为化纤织物和醋酸纤维素的添加剂,除自熄性外,还改善了耐水性和抗静电性,一般用量为5~10份。
此外,磷酸三(2-氯乙基)酯(TCEP)广泛用于醋酸纤维素、硝基纤维素、乙基纤维素、聚氯乙烯、聚氨酯、聚醋酸乙烯和酚醛树脂中。作为阻燃性增塑剂时,它还可用作金属萃取剂、润滑油和汽油添加剂,以及聚酰亚胺加工改性剂。大鼠口毒性LD50为1410 mg/kg。
生产方法TCEP的制备通常采用两种方法:一种是三氯氧磷与环氧乙烷在偏钒酸钠催化剂下于50℃反应,再经中和、水洗、真空脱水及脱低沸物处理;另一种是以氯乙醇为原料,与三氯氧磷或三氯化磷反应制造磷酸三(2-氯乙基)酯。
具体步骤如下:将326 kg三氯氧磷和1.0 kg偏钒酸钠投入反应釜中。充氮驱除空气后,在真空条件下通入650 kg环氧乙烷,于45~50°C下搅拌2~3小时。随后蒸出过量的环氧乙烷并加碱中和至中性,水洗,真空脱水得成品。
类别TCEP属于有毒物质,毒性分级为中毒。急性毒性表现为:口服-大鼠 LD50: 1230 毫克/公斤;口服-小鼠 LD50: 1866 毫克/公斤。刺激数据包括皮肤轻微反应(兔,10毫克/24小时)和眼睛轻度刺激(兔,500毫克/24小时)。该物质可燃,在火场分解产生有毒氯化氢和磷氧化物气体。
储运特性储存于低温、通风及干燥的库房中。
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三(2-氯乙基)亚磷酸酯 tris(2-chloro-ethyl)phosphite 140-08-9 C6H12Cl3O3P 269.492 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— phosphoric acid bis-(2-hydroxy-ethyl) ester 18924-97-5 C4H11O6P 186.101
反应信息
-
作为反应物:参考文献:名称:Korschak et al., Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1958, p. 210,215; engl. Ausg. S. 196, 200摘要:DOI:
-
作为产物:参考文献:名称:负载路易斯酸性离子液体催化的环氧醚裂解反应摘要:用路易斯酸性离子液体催化环氧丙烷与POCl 3的反应。考虑到较低的成本和催化活性,我们得出结论,从经济角度来看,[Et 3 NH] Cl / AlCl 3是最具吸引力的离子液体。但是由于对水和空气敏感,很容易使其失活。而且,由于反应中的回收困难,它不能容易地重复使用。然而,负载[Et 3 NH] Cl / AlCl 3催化剂可以解决上述问题。负载[Et 3 NH] Cl / AlCl 3催化剂可以很容易地通过过滤器分离,并可以以98%的产率重复使用5次。此外,该催化剂可用于其他环氧醚裂解反应。DOI:10.1016/j.cclet.2012.09.015
-
作为试剂:参考文献:名称:Kormachev, V. V.; Solodova, K. V.; Vorozheva, T. N., Journal of general chemistry of the USSR, 1984, vol. 54, p. 895 - 898摘要:DOI:
文献信息
-
[EN] HYDROSILYLATION REACTION CURABLE COMPOSITIONS AND METHODS FOR THEIR PREPARATION AND USE<br/>[FR] COMPOSITIONS DURCISSABLES PAR UNE RÉACTION D'HYDROSILYLATION ET LEURS PROCÉDÉS DE PRÉPARATION ET D'UTILISATION申请人:DOW CORNING公开号:WO2013000788A1公开(公告)日:2013-01-03A composition contains (A) a hydrosilylation reaction catalyst and (B) an aliphatically unsaturated compound having an average, per molecule, of one or more aliphatically unsaturated organic groups capable of undergoing hydrosilylation reaction. The composition is capable of reacting via hydrosilylation reaction to form a reaction product, such as a silane, a gum, a gel, a rubber, or a resin. Ingredient (A) contains a platinum-ligand complex that can be prepared by reacting a platinum precursor and a ligand.
-
[EN] ACRYLATE-FUNCTIONAL BRANCHED ORGANOSILICON COMPOUND, METHOD OF PREPARING SAME, AND COPOLYMER FORMED THEREWITH<br/>[FR] COMPOSÉ D'ORGANOSILICIUM RAMIFIÉ À FONCTION ACRYLATE, SON PROCÉDÉ DE PRÉPARATION ET COPOLYMÈRE FORMÉ AVEC CELUI-CI申请人:DOW SILICONES CORP公开号:WO2020142474A1公开(公告)日:2020-07-09A method of preparing an acrylate-functional branched organosilicon compound ("compound") is provided, and comprises reacting (A) a branched organosilicon compound and (B) an acrylate compound in the presence of (C) a catalyst, wherein component (A) has the general formula X-Si(R1)3, where X comprises a halogen-functional moiety and each R1 is selected from R and –OSi(R4)3, with the proviso that at least one R1 is –OSi(R4)3; each R4 is selected from R, –OSi(R5)3, and –[OSiR2]mOSiR3; each R5 is selected from R, –OSi(R6)3, and –[OSiR2]mOSiR3; each R6 is selected from R and –[OSiR2]mOSiR3; each R is an independently selected hydrocarbyl group; and 0≤m≤100; with the proviso that at least one of R4, R5 and R6 is –[OSiR2]mOSiR3. The compound prepared by the method, a copolymer comprising the reaction product of the compound and a second compound, a method of forming the copolymer, and a composition comprising the copolymer are each also provided.提供了一种制备丙烯酸酯官能化分支有机硅化合物(“化合物”)的方法,包括在催化剂存在下,使(A)分支有机硅化合物和(B)丙烯酸酯化合物发生反应,其中组分(A)具有一般式X-Si(R1)3,其中X包括卤素官能基,每个R1从R和–OSi(R4)3中选择,但至少一个R1为–OSi(R4)3;每个R4从R、–OSi(R5)3和–[OSiR2]mOSiR3中选择;每个R5从R、–OSi(R6)3和–[OSiR2]mOSiR3中选择;每个R6从R和–[OSiR2]mOSiR3中选择;每个R是独立选择的烃基;且0≤m≤100;但至少一个R4、R5和R6为–[OSiR2]mOSiR3。还提供了通过该方法制备的化合物,包括化合物和第二化合物的反应产物的共聚物,形成共聚物的方法,以及包含共聚物的组合物。
-
NITROGEN-CONTAINING COMPOUNDS SUITABLE FOR USE IN THE PRODUCTION OF POLYURETHANES申请人:Evonik Degussa GmbH公开号:US20180194889A1公开(公告)日:2018-07-12The present invention provides for the use of nitrogen compounds of formula (I) and/or of corresponding quaternized and/or protonated compounds for production of polyurethanes, compositions containing these compounds and polyurethane systems, especially polyurethane foams, which have been obtained using the compounds.
-
Hydrosilylation Reaction Catalysts and Curable Compositions and Methods for Their Preparation and Use申请人:Dow Corning Corporation公开号:US20140296468A1公开(公告)日:2014-10-02A composition contains (A) a hydrosilylation reaction catalyst and (B) an aliphatically unsaturated compound having an average, per molecule, of one or more aliphatically unsaturated organic groups capable of undergoing hydrosilylation reaction. The composition is capable of reacting via hydrosilylation reaction to form a reaction product, such as a silane, a gum, a gel, a rubber, or a resin. Ingredient (A) contains an iron-organosilicon ligand complex that can be prepared by reacting an iron carbonyl compound and an organosilicon ligand.
-
AMIDINE GROUP - OR GUANIDINE GROUP - CONTAINING SILANE申请人:SIKA TECHNOLOGY AG公开号:US20170081348A1公开(公告)日:2017-03-23A silane of the formula (I) containing at least one aliphatic amidine group- or guanidine group-containing alkoxy group, to a method for producing same, to conversion products thereof, and to the use thereof as a catalyst in curable compositions, in particular based on silane group-containing polymers. The silane of the formula (I) is largely odorless and non-volatile at room temperature. The silane accelerates the hydrolysis and condensation reaction of silane groups very effectively without impairing the storage stability of silane group-containing polymers. Additionally, the silane is very tolerable in silane group-containing compositions, whereby such compositions are not prone to separate, migrate, or evaporate the catalyst.
表征谱图
-
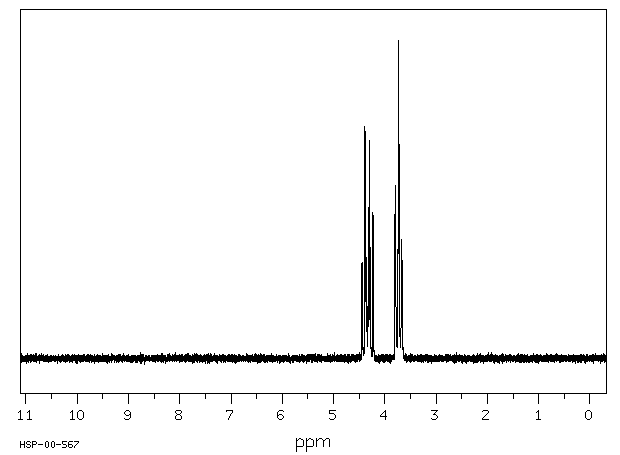
氢谱1HNMR
-
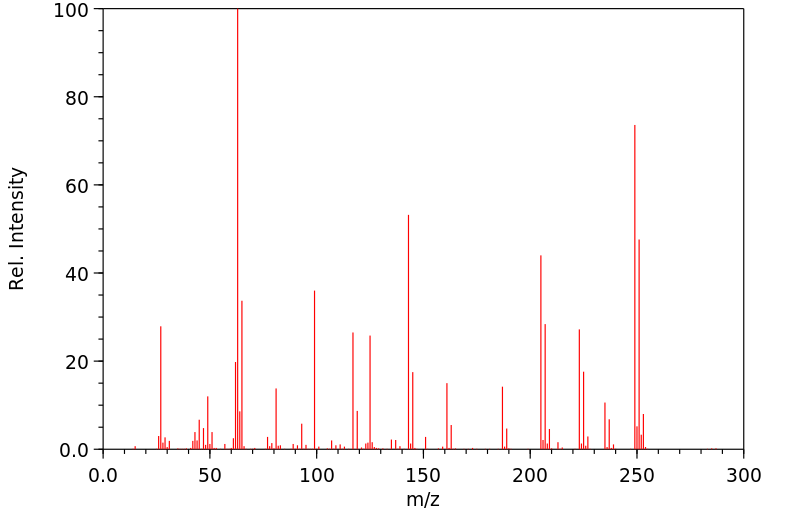
质谱MS
-
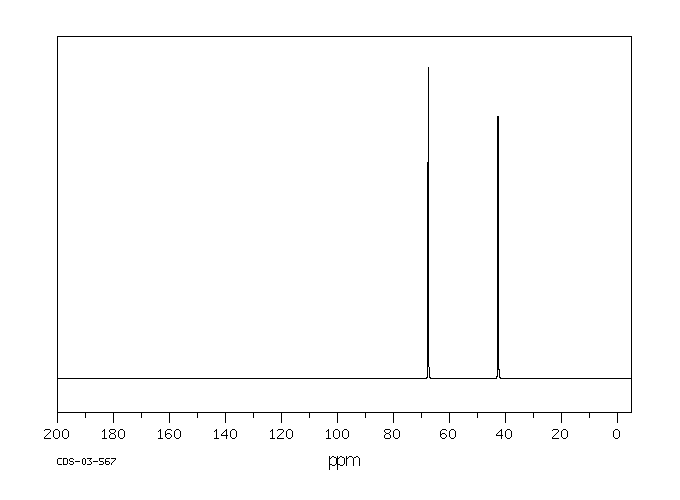
碳谱13CNMR
-
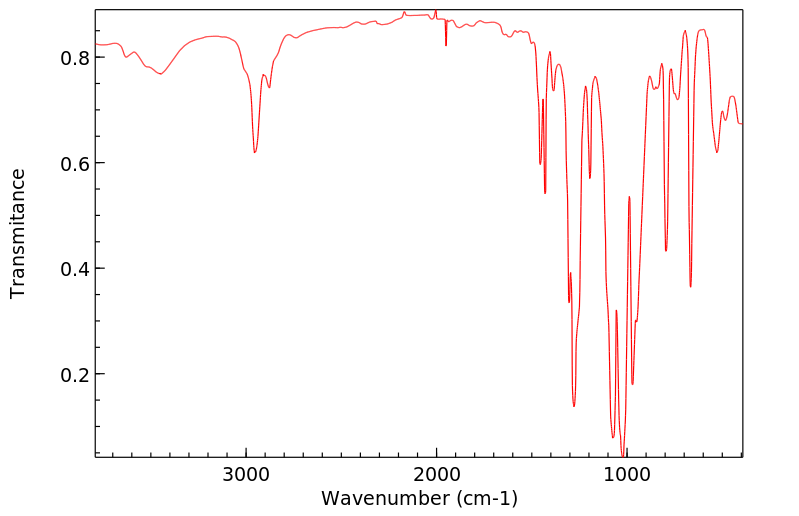
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息