2,2-二甲基-1,3-二噻戊环 | 6008-78-2
中文名称
2,2-二甲基-1,3-二噻戊环
中文别名
——
英文名称
2,2-dimethyl-[1,3]dithiolane
英文别名
2,2-Dimethyl-[1,3]dithiolan;2,2-Dimethyl-1,3-dithiolane
CAS
6008-78-2
化学式
C5H10S2
mdl
——
分子量
134.266
InChiKey
HEDYXMHGMMWNBO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:48 °C(Press: 6-7 Torr)
-
密度:1.064±0.06 g/cm3(Predicted)
-
保留指数:1032
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Griller, David; Nonhebel, Derek C.; Walton, John C., Journal of the Chemical Society. Perkin transactions II, 1984, # 11, p. 1817 - 1822摘要:DOI:
-
作为产物:描述:参考文献:名称:Robbe; Fernandez; Dubief, European Journal of Medicinal Chemistry, 1982, vol. 17, # 3, p. 235 - 243摘要:DOI:
文献信息
-
Trichloroisocyanuric Acid as a Mild and Efficient Catalyst for Thioacetalization and Transthio-acetalization Reactions作者:H. Firouzabadi、N. Iranpoor、H. HazarkhaniDOI:10.1055/s-2001-17451日期:——Trichloroisocyanuric acid (1), a cheap industrial chemical, catalyzes mild and efficient thioacetalization and transthioacetalization reactions. In addition, this catalyst is very selective for this purpose.
-
The reactivity of (Me3Si)3SiH with sulfoxides under free radical conditions作者:Chryssostomos Chatgilialoglu、Carla FerreriDOI:10.1016/j.tet.2016.05.080日期:2016.12radical-initiated reaction of (Me3Si)3SiH with a variety of sulfoxides has been investigated. The reactivity varies significantly with the nature of the starting materials. The reactions of diaryl sulfoxides provide the corresponding sulfides in high yields while the related reactions with dialkyl sulfoxides occur more slowly and in moderate yields. The rate constant for the addition of (Me3Si)3Si radical with研究了(Me 3 Si)3 SiH与多种亚砜的自由基引发反应。反应性随原料的性质而显着变化。二芳基亚砜的反应以高收率提供相应的硫化物,而与二烷基亚砜的相关反应则以较慢的速度和中等收率发生。在80°C下,将(Me 3 Si)3 Si自由基与二丁基亚砜加成的速率常数在10 3 –10 4 M -1 s -1范围内。(Me 3 Si)3的反应活性在比较研究中还研究了具有1,3-二硫杂环戊烷衍生物的SiH。2,2-二甲基-1,3-二硫杂环戊烷和相应的1,1-二氧化物与(Me 3 Si)3 Si自由基在硫原子上的攻击非常平滑,然后在S–CMe 2键处开环,随后发生H -从硅烷中以非常高的收率进行萃取,而2,2-二甲基-1,3-二硫杂环戊烷-1-氧化物的行为则完全不同且出乎意料。
-
The Reaction of 2-Ethoxy-1,3-dithiolane with Carbonyl Compounds作者:Shigeo Jo、Shigeo Tanimoto、Tatsuo Oida、Masaya OkanoDOI:10.1246/bcsj.54.1434日期:1981.5The reaction of 2-ethoxy-1,3-dithiolane with carbonyl compounds such as aldehydes and ketones was investigated. The reaction proceeded smoothly in the presence of the HgCl2-catalyst to afford 2-substituted and 2,2-disubstituted 1,3-dithiolanes. The reaction also offers an interesting alternative to the previously reported methods of synthesizing 1,3-dithiolanes which involve the acid-catalyzed reaction of carbonyl compounds with 1,2-ethanedithiol.
-
Selective Transdithioacetalization of Acetals, Ketals, Oxathioacetals and Oxathioketals Catalyzed by Envirocat EPZ10R作者:A. S. Gajare、M. S. Shingare、B. P. BandgarDOI:10.1039/a800864g日期:——Envirocat EPZ10R has been found to be a remarkable reusable heterogeneous catalyst for selective transdithioacetalization of acetals, ketals, oxathioacetals and oxathioketals with HSCH2CH2SH and HSCH2CH2CH2SH.
-
Anodic oxidation of dialkyl and diaryl dithioacetals作者:Jean Gourcy、Patrick Martigny、Jacques Simonet、Georges JeminetDOI:10.1016/s0040-4020(01)92089-7日期:1981.1The mechanism of the anodic oxidation of dithioacetals is discussed, taking into account new results concerning both mixed electrolyses and oxidations in super-dried solvents. In the case of the oxidation of aryldithioacetals, the formation of the -S-S- linkage is involved with a bond cleavage followed by a dimerisation. On the other hand, in the case of aliphatic starting materials the mechanism looks
表征谱图
-
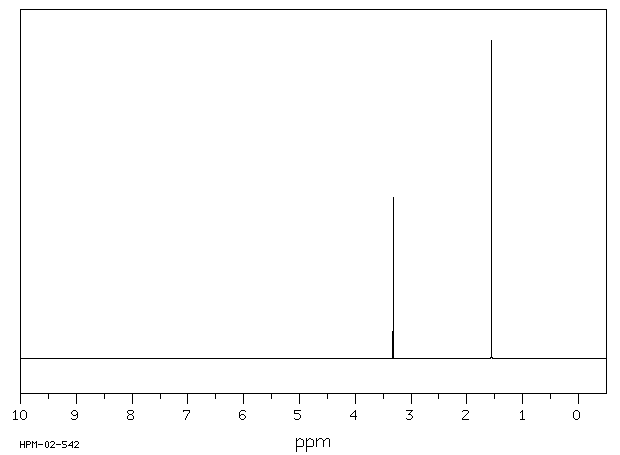
氢谱1HNMR
-
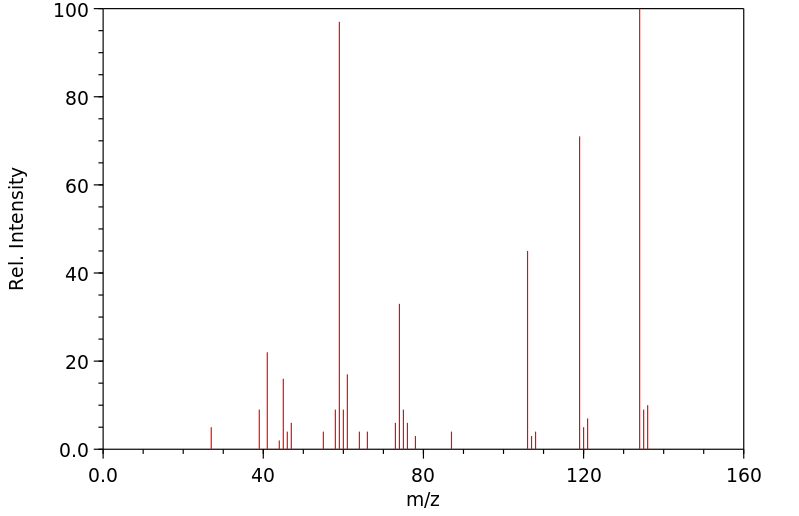
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
雷尼替丁EP杂质J
苯乙酮乙烷-1,2-二基二硫代缩醛
苯丙酮乙烷-1,2-二基二硫代缩醛
磷亚胺酸,[2,3,4,5,6-五氯-2,3,5,6-四氟-1-(2,2,3,3-四氟丙氧基)-4-(三氟甲基)环己基]-,三(2,2,3,3-四氟丙基)酯
硫代磷酸O,O-二乙基S-[2,2-二(乙硫基)丙基]酯
硫代二碳酸叔丁基乙基酯
硫代二碳酸 1-乙基 3-异丙基酯
甲硫基甲酸叔丁酯
甲氧基甲基硫烷基乙烷
甲氧基二硫代甲酸甲酯
甲氧基(甲基硫烷基)甲烷
甲基二[[(二甲基氨基)硫代甲酰]硫代]乙酸酯
甲基8-氧代-6,10-二硫杂螺[4.5]癸烷-7-羧酸酯
环辛酮硫代缩酮
环线威
环己基甲硫基甲基醚
环己基二乙酸二乙酯
氰硫基酸,2,2,2-三氯乙基酯
双(硫代甲氧基甲基)硫醚
双(亚甲基二硫代)四硫富瓦烯
六氢-2'3A-二甲基螺[1,3-二硫环戊并[4,5-B]呋喃-2,3'(2'H)-呋喃]
亚甲基二(氰基亚胺硫代碳酸甲酯)
亚甲基二(二异丁基二硫代氨基甲酸酯)
二邻茴香醚
二硫氰基甲烷
二硫代丁酸甲酯
二甲硫基甲烷
二甲氧基-[(2-甲基-1,3-氧硫杂环戊烷-2-基)甲硫基]-巯基膦烷
二异丙基黄原酸酯
二(硫代碳酸 O-丁基酯)硫代酸酐
二(二甲基二硫代氨基甲酸)亚甲基酯
二(乙硫基)甲烷
二(乙硫基)乙酸乙酯
二(乙氧基硫代羰基)硫醚
二(2-氨基乙基硫基)甲烷
乙醛,二(甲硫基)-
乙酸甲硫甲酯
乙氧基甲基异硫脲盐酸盐
乙丙二砜
乙丁二砜
丙烷-2、2-二基双(磺胺二基)二乙胺
丙烷-2,2-二基双(硫)基]二乙酸
三硫丙酮
[(异丙氧基硫基甲酰基硫基)硫基甲酰基硫基]硫代甲酸O-异丙基酯
[(N,N-二甲基二硫代氨基甲酰)甲基]甲基氰基亚氨二硫代碳酸酯
[(2R,4S,6R)-4,6-二甲基-1-硫羟基-1,3-二硫烷-2-基](二苯基)磷烷
[(2-羧基乙氧基)甲基]二甲基-锍溴化物(1:1)
S-甲基O-(2-甲基丙基)二硫代碳酸酯
S-烯丙基 O-戊基二硫代碳酸酯
S-乙基O-(1-碘乙基)硫代碳酸酯