2,3,5,6-四氯酚 | 935-95-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:115 °C
-
沸点:261.0±35.0 °C(Predicted)
-
密度:1.6 g/cm3(Temp: 60 °C)
-
物理描述:Leaflets (from ligroin) or light beige powder. (NTP, 1992)
-
颜色/状态:Leaf, from ligroin
-
溶解度:less than 1 mg/mL at 68° F (NTP, 1992)
-
蒸汽密度:Relative vapor density (air = 1): 8.0
-
蒸汽压力:Vapor pressure, Pa at 100 °C: 130
-
分解:288 °C
-
解离常数:pKa = 5.14
-
保留指数:1515;1536;1545;1530;1511;1530;1551;1511
-
稳定性/保质期:
常温常压下稳定,避免酸酐与酰基氯接触以防止氧化。
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
安全信息
-
危险等级:6.1
-
危险品标志:F,T
-
安全说明:S16,S26,S36/37,S39,S45
-
危险类别码:R11,R37/38,R23/24/25,R39/23/24/25,R25,R41
-
WGK Germany:3
-
危险品运输编号:UN 2020 6.1/PG 3
-
RTECS号:SM9450000
-
海关编码:2908199090
-
包装等级:III
-
危险类别:8,6.1
-
危险性防范说明:P261,P280,P301+P310,P305+P351+P338
-
危险性描述:H301,H315,H318,H335,H413
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 2,3,5,6-四氯苯酚 |
| 化学品英文名称: | 2,3,5,6-Tetrachloropheno1 |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 935-95-5 |
| 分子式: | C 6 H 2 Cl 4 O |
| 分子量: | 231.88 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:2,3,5,6-四氯苯酚 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 本品有毒。对眼睛、皮肤、粘膜和上呼吸道有刺激作用,可致眼睛损伤。受热分解放出有毒的氯气。 |
| 环境危害: | 对环境有危害。 |
| 燃爆危险: | 本品可燃,有毒,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。呼吸困难时给输氧。呼吸停止时,立即进行人工呼吸。就医。 |
| 食入: | 误服者,口服牛奶、豆浆或蛋清,就医。 |
| 第五部分:消防措施 |
| 危险特性: | 不易燃烧。受高热分解,放出有毒的烟气。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳、氯化氢。 |
| 灭火方法及灭火剂: | 消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。灭火剂:抗溶性泡沫、二氧化碳、干粉。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好防毒面具,穿化学防护服。用砂土、干燥石灰或苏打灰混合,收集于一个密闭的容器中,运至废物处理场所。用水刷洗泄漏污染区,对污染地带进行通风。如大量泄漏,收集回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,局部排风。防止粉尘释放到车间空气中。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防尘口罩,戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂、酸酐、酰基氯接触。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。防止阳光直射。包装密封。应与氧化剂、酸酐、酰基氯、食用化学品分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 密闭操作,局部排风。 |
| 呼吸系统防护: | 佩戴防尘口罩。空气中浓度较高时,建议佩戴防毒面具。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿相应的防护服。 |
| 手防护: | 戴防化学品手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋浴更衣。保持良好的卫生习惯。特别注意眼和呼吸道的防护。 |
| 第九部分:理化特性 |
| 外观与性状: | 灰白色至褐色粉末。 |
| pH: | |
| 熔点(℃): | 114~116 |
| 沸点(℃): | 164/3.059kPa |
| 相对密度(水=1): | 1.6(60/4℃) |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 6 H 2 Cl 4 O |
| 分子量: | 231.88 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 微溶于水,易溶于醇、醚、苯。 |
| 主要用途: | 用作杀菌剂和木材防腐剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 氧化剂、酸酐、酰基氯。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氯化氢。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:109mg/kg(小鼠经口) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 建议用焚烧法处置。与易燃溶剂混合后,再焚烧。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。保持容器密封。防止阳光曝晒。专人保管。操作现场不得吸烟、饮水、进食。应与氧化剂分开存放。不能与粮食、食物、种子、饲料、各种日用品混装、混运。搬运时要轻装轻卸,防 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 6 |
| MSDS修改日期: | 年月日 |
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 五氯酚 Pentachlorophenol 87-86-5 C6HCl5O 266.338 2,3,5,6-四氯茴香醚 2,3,5,6-tetrachloroanisole 6936-40-9 C7H4Cl4O 245.92 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,3,6-三氯苯酚 2,3,6-trichlorophenol 933-75-5 C6H3Cl3O 197.448 2,3,5-三氯苯酚 2,3,5-trichlorophenol 933-78-8 C6H3Cl3O 197.448 四氯氰醌 2,3,5,6-tetrachlorobenzene-1,4-diol 87-87-6 C6H2Cl4O2 247.893 二氯-苯酚 2,3-dichlorophenol 576-24-9 C6H4Cl2O 163.003 2,3,5,6-四氯茴香醚 2,3,5,6-tetrachloroanisole 6936-40-9 C7H4Cl4O 245.92 2,6-二氯苯酚 2,6-Dichlorophenol 87-65-0 C6H4Cl2O 163.003 —— 2,2',3,3',5,6-Hexachlorodiphenyl ether 727738-90-1 C12H4Cl6O 376.9 —— 4-bromo-2,3,5,6-tetrachlorophenol 4091-48-9 C6HBrCl4O 310.789 —— 2,3,3',4',5,6-Hexachlorobiphenyl ether 155999-97-6 C12H4Cl6O 376.881 1,2,4,5-四氯-3-(2,4-二氯苯氧基)苯 2,2',3,4',5,6-hexachlorobiphenyl ether 116995-18-7 C12H4Cl6O 376.881 1,2,4,5-四氯-3-(2,4,5-三氯苯氧基)苯 2,2',3,,4',5,5',6-heptachlorodiphenylether 109828-23-1 C12H3Cl7O 411.326 1,2,4,5-四氯-3-(2,3,4-三氯苯氧基)苯 2,2',3,3',4',5,6-heptachlorodiphenylether 83992-71-6 C12H3Cl7O 411.326 2,3,5,6-四氯苯酚乙酸酯 2,3,5,6-Tetrachlorophenyl acetate 61925-90-4 C8H4Cl4O2 273.9 邻氯苯酚 2-Chlorophenol 95-57-8 C6H5ClO 128.558 —— Tetrachloro-4-hydroxybenzaldehyde 83016-60-8 C7H2Cl4O2 259.904 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:鉴定人血浆中的羟基化PCB代谢物和其他酚类卤化污染物。摘要:越来越多的研究报告了保留在人类和野生生物血液中的酚类卤代化合物(PHC)。这些初级化学品可能是工业化学品。代谢产物,如聚氯联苯酚(OH-PCBs);或自然起源。本研究旨在鉴定迄今未知的人血浆中具有与已知具有内分泌干扰活性的PHC一致的化学结构的PHC。为此,将来自瑞典的10位随机选择的男性献血者的血浆样本汇总起来并通过GC / ECD和GC / MS进行分析。合成了苯酚和OH-PCBs的溴代,溴代氯代和氯代甲基衍生物,以用作可靠的参考标准品。血浆中指示有100多种PHC,在这些化合物中,总共鉴定出9种单环溴化或氯化酚,愈创木酚和/或儿茶酚型化合物为它们的甲基化衍生物。两种主要化合物是2,4,6-三溴苯酚和五氯苯酚。在两个极性不同的气相色谱柱上,在结构上鉴定出38个OH-PCB同类物。建议在其母体PCB同系物的背景下OH-PCB代谢物的起源。在男性血浆中鉴定出的其他PHC是三氯生(5-氯-2-DOI:10.1007/s002440010298
-
作为产物:描述:参考文献:名称:苯偶氮基团将氯和氟活化为亲核芳族取代基。区域选择性制备多取代苯胺摘要:使用苯基偶氮基团将邻氟和氯原子选择性活化为丙硫醇盐阴离子进行亲核芳族取代。这实现了三个取代的4-烷氧基苯胺的区域选择性合成。通过将实验NMR化学位移与经验预测值进行比较,可以确定取代的区域选择性。在取代基效应的背景下讨论了所观察到的底物的反应性。DOI:10.1016/j.tet.2005.01.023
文献信息
-
Room-Temperature Copper-Catalyzed Oxidation of Electron-Deficient Arenes and Heteroarenes Using Air作者:Qiang Liu、Pan Wu、Yuhong Yang、Ziqi Zeng、Jie Liu、Hong Yi、Aiwen LeiDOI:10.1002/anie.201200750日期:2012.5.7No pressure: The oxidation of aromatic CH bonds at room temperature was realized through a copper‐catalyzed “oxygenase‐type” oxidation of arenes and heteroarenes in the presence of air (see scheme). The reaction involves an oxygen‐atom transfer from O2 in the air onto the substrates.
-
3-(PHENOXYPHENYLMETHYL)PYRROLIDINE COMPOUNDS申请人:Stangeland Eric公开号:US20100022616A1公开(公告)日:2010-01-28In one aspect, the invention relates to compounds of formula I: where a and R 1-6 are as defined in the specification, or a pharmaceutically acceptable salt thereof. The compounds of formula I are serotonin and norepinephrine reuptake inhibitors. In another aspect, the invention relates to pharmaceutical compositions comprising such compounds; methods of using such compounds; and process and intermediates for preparing such compounds.
-
Electroreduction of Organic Compounds, 34 [1]. Cathodic Dehalogenation of Chloroarenes with Electron-Donating Substituents作者:Olaf Kranz、Jürgen VossDOI:10.1515/znb-2003-1206日期:2003.12.1
The electrochemical reduction of chlorinated arenes with electron-donating substituents, i. e. chlorotoluenes, -anisoles and -phenols, is studied. Preparative electrolyses are run in various solventsupporting electrolytes under potentiostatic and galvanostatic conditions at lead or carbon cathodes. A partial and mostly regioselective hydrodechlorination of compounds with two or more chloro substituents is possible under suitable conditions. The replacement of one single chloro substituent, in particular in a para-position, is difficult. Highly toxic and persistent oligochloro derivatives are thus transformed into less problematic compounds with a low degree of chlorination. The chlorine content of real-life materials such as extracts of soil contaminated with chlorinated phenols and Nitrofen® can also be significantly decreased by electroreduction.
-
SYNTHESIS OF CLAY-TEMPLATED SUBNANO-SIZED ZERO VALENT IRON (ZVI) PARTICLES, CLAYS CONTAINING SAME, AND USE OF BOTH IN CONTAMINANT TREATMENTS申请人:Li Hui公开号:US20110130575A1公开(公告)日:2011-06-02A clay comprising a 2:1 aluminosilicate clay having negative charge sites, the 2:1 aluminosilicate clay containing subnano-sized zero valent iron (ZVI) particles distributed on clay surfaces is provided. In one embodiment, at least some or all of the particles have a cross-section of five ( 5 ) angstroms or less. Methods of synthesizing and the novel clays and the clay-templated subnano-scale ZVI particles themselves are also described. Such novel products are useful in a variety of remediation applications, including for reduction and dechlorination reactions.
-
Structural diversity of hydrogen-bonded complexes comprising phenol-based and pyridine-based components: NLO properties and crystallographic and spectroscopic studies作者:Iwona Bryndal、Marek Drozd、Tadeusz Lis、Jan K. Zaręba、Henryk RatajczakDOI:10.1039/d0ce00606h日期:——transfer is found, attesting to its co-crystal nature. The 2,5-DNP molecule was found to possess a short intramolecular O–H⋯O hydrogen bond and crystallize in the monoclinic crystal system with space group P21. Crystal structure analysis reveals that the substituted phenol–pyridine complexes crystallize as salts linked by N–H⋯O hydrogen bonds in the orthorhombic crystal system with space group Pna21, (1)具有改善的物理化学性质的化合物的合成,包括非线性光学现象,例如二次谐波的产生,可能被认为是晶体工程最具挑战性的方面之一。在这项工作中,我们检查了氢键网络的多样性和在包含酚基氢键供体(2,5-二硝基苯酚,4-硝基苯酚,2,3,5,6-四氯苯酚)的新型非中心对称配合物中的二次谐波产生的相对效率。 )和基于吡啶鎓的氢键受体(2-氨基吡啶,3,5-二甲基吡啶,2,3,6-三甲基吡啶):2-氨基吡啶2,5-二硝基苯酚盐[(2APy)+ ·(2,5-DNP)- ](1),4-氨基苯酚2-氨基吡啶鎓4-硝基苯酚[(2APy)+·(4-NP)− ·(4-NP)](2),2,3,6-三甲基吡啶2,3,5,6-四氯酚盐[(2,3,6C)+ ·(TCP)− ](3)和3,5-二甲基吡啶2,3,5,6-四氯苯酚[(3,5L)·(TCP)](4)。在化合物(1)-(3)中,质子转移发生;具体地,化合物(1)和(3)
表征谱图
-
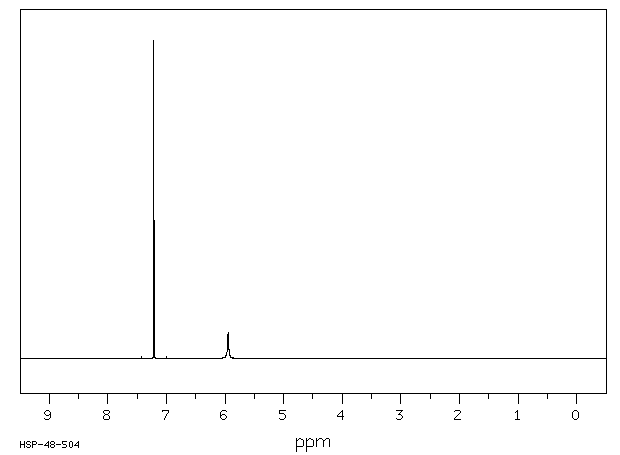
氢谱1HNMR
-
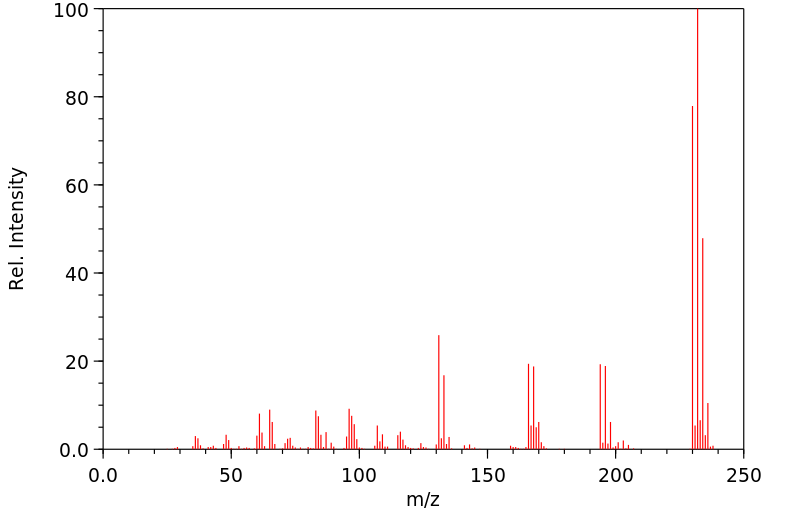
质谱MS
-
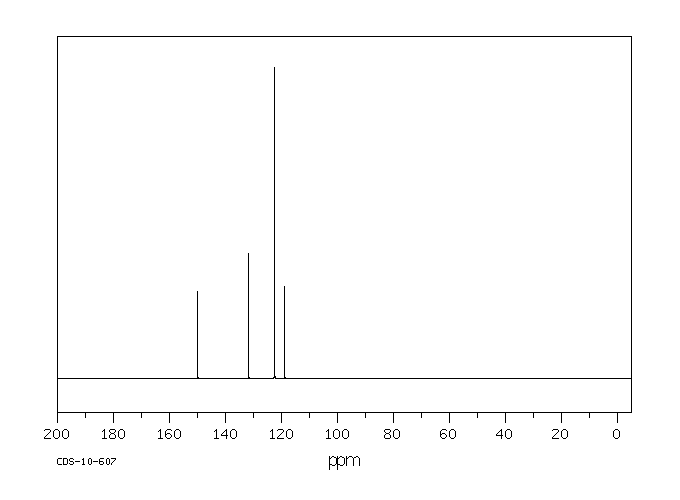
碳谱13CNMR
-
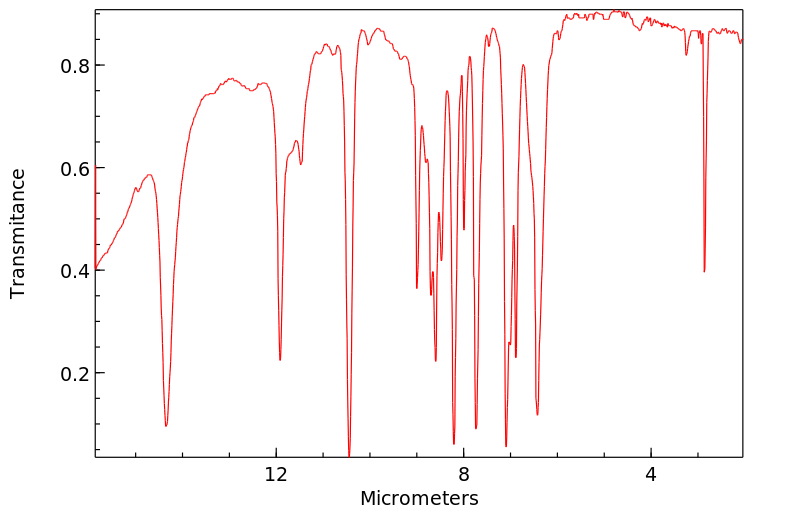
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息