2,3-二溴-4-羟基-5-甲氧基苯甲醛 | 2973-75-3
中文名称
2,3-二溴-4-羟基-5-甲氧基苯甲醛
中文别名
——
英文名称
5,6-dibromovanillin
英文别名
2,3-dibromo-4-hydroxy-5-methoxybenzaldehyde
CAS
2973-75-3
化学式
C8H6Br2O3
mdl
MFCD00016978
分子量
309.942
InChiKey
WKLKGSHBXNPUDU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:212-215 °C
-
密度:1.9544 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2913000090
-
储存条件:存储于阴凉干燥处
SDS
| Name: | 2 3-Dibromo-4-hydroxy-5-methoxybenzaldehyde Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2973-75-3 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2973-75-3 | 2,3-Dibromo-4-hydroxy-5-methoxybenzald | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Air sensitive.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2973-75-3: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 212 - 215 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H6Br2O3
Molecular Weight: 309.94
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2973-75-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,3-Dibromo-4-hydroxy-5-methoxybenzaldehyde - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 2973-75-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2973-75-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2973-75-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-溴香兰素 5-bromo-4-hydroxy-3-methoxybenzaldehyde 2973-76-4 C8H7BrO3 231.046 6-溴香兰素 6-bromovanillin 60632-40-8 C8H7BrO3 231.046 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,3-dibromo-4,5-dimethoxybenzaldehyde 70625-29-5 C9H8Br2O3 323.969 2,3-二溴-4,5-二羟基苯甲醛 2,3-dibromo-4,5-dihydroxybenzaldehyde 14045-41-1 C7H4Br2O3 295.915 —— 2,3-dibromo-5-methoxy-4-((4-methylphenyl)methoxy)benzaldehyde —— C16H14Br2O3 414.093 2,3-二溴-4-(羟甲基)-6-甲氧基苯酚 2,3-Dibrom-4-hydroxy-5-methoxy-benzylalkohol 1940-81-4 C8H8Br2O3 311.958 —— 2,3-dibromo-4-hydroxy-5-methoxybenzonitrile 330462-54-9 C8H5Br2NO2 306.941 —— 5.6-dibromo-4-hydroxy-3-methoxy-trans-cinnamic acid 61223-34-5 C10H8Br2O4 351.979 2,3-二溴-4,5-二羟基苄醇 lanosol 4950-06-5 C7H6Br2O3 297.931 —— 3,4-dibromo-5-(methoxymethyl)benzene-1,2-diol 14045-42-2 C8H8Br2O3 311.958 —— 3,4-dibromo-5-(ethoxymethyl)benzene-1,2-diol 54502-93-1 C9H10Br2O3 325.985 —— 2.3-Dibrom-4.5-dihydroxy-benzyl-propylaether 52897-62-8 C10H12Br2O3 340.011 —— (Z)-5-(2,3-dibromo-4-hydroxy-5-methoxybenzylidene)imidazolidine-2,4-dione —— C11H8Br2N2O4 392.004 - 1
- 2
反应信息
-
作为反应物:描述:2,3-二溴-4-羟基-5-甲氧基苯甲醛 以79%的产率得到2,3-dibromo-4,5-dimethoxybenzaldehyde参考文献:名称:Cephem compounds, and antibacterial agents摘要:本发明揭示了由公式[I]表示的新型头孢菌素化合物及其药理学上可接受的盐或可在生理上水解的无毒酯类。其中,R1表示直链或支链低碳基,可以被保护的羧基取代,三苯甲基基团,氢原子或氟代低碳基;R2表示氢原子,金属原子,羧基保护基或可在体内产生水解酯残基的酯残基;R3、R4、R5和R6可以相同也可以不同,表示氢原子,卤素原子,直链或支链低碳基,可以被取代的巯基,可以被取代的低碳基氨基,可以被保护的羟基,低碳基氧基,低碳基酰基,低碳基氧羰基,或者可以被取代的低碳基亚甲二氧基基团,可以与R3和R4形成;R7表示氢原子,氰基,卤素原子或COOR8(R8为氢或低碳基);R9表示可以被保护的氨基;Z表示N或CH;n表示0或1。本发明还声明了它们的制备过程以及含有它们作为有效成分的抗菌剂。公开号:US05244892A1
-
作为产物:描述:参考文献:名称:发现新型溴酚-硫代氨基脲杂化物作为用于癌症的聚(ADP-核糖)聚合酶-1(PARP-1)的强效选择性抑制剂。摘要:聚(ADP-核糖)聚合酶-1(PARP-1)是抗癌药物发现的新潜在目标。设计,合成并评估了一系列作为PARP-1抑制剂的溴酚-硫代半碳杂zone杂化物的抗肿瘤活性。其中,最有前途的化合物11对PARP-2(IC50> 1000 nM)表现出优异的选择性PARP-1抑制活性(IC50 = 29.5 nM),并对SK-OV-3,Bel-7402和在体内SK-OV-3细胞异种移植模型中,HepG2癌细胞系(IC50 = 2.39、5.45和4.60μM)以及肿瘤生长的抑制作用。进一步的研究表明,化合物11通过多种抗癌机制发挥了抗肿瘤作用,包括诱导凋亡和细胞周期停滞,DNA双链断裂的细胞蓄积,DNA修复改变,抑制H2O2触发的PARylation,通过产生细胞毒性活性氧而产生的抗增殖作用以及自噬。另外,化合物11显示出良好的药代动力学特性和良好的安全性。这些观察表明,化合物11可以用作发现新的抗癌药物的先导化合物。DOI:10.1021/acs.jmedchem.8b01946
文献信息
-
NOVEL CURCUMIN DERIVATIVE申请人:Sugimoto Hachiro公开号:US20110082295A1公开(公告)日:2011-04-07To develop a highly safe measure to treat Alzheimer's disease using a secretase-inhibiting substance, there is provided a compound represented by the following general formula (I) or a salt thereof: wherein A represents a phenyl group or the like, R 1 represents a chlorine atom, a bromine atom, or a nitro group or the like, R 2 , R 3 , R 4 , and R 5 each represent a hydrogen atom or the like, and L represents CH 2 —CH 2 or CH═CH.
-
Direct Access to Monoprotected Homoallylic 1,2-Diols via Dual Chromium/Photoredox Catalysis作者:Felix Schäfers、Linda Quach、J. Luca Schwarz、Mar Saladrigas、Constantin G. Daniliuc、Frank GloriusDOI:10.1021/acscatal.0c03697日期:2020.10.16Herein, we present a dual catalytic strategy to efficiently obtain monoprotected homoallylic 1,2-diols by coupling abundant aldehydes with simple (silyl) enol ethers, thus providing direct access to this important motif without the (super) stoichiometric use of prefunctionalized metal-allyl species. The modularity of our approach is shown by the introduction of several silyl- and alkyl-based protecting
-
溴酚-噁唑类化合物及其在治疗2型糖尿病药物中的应用
-
Decarboxylative C<sub>sp3</sub>–C<sub>sp3</sub> coupling for benzylation of unstable ketone enolates: synthesis of p-(acylethyl)phenols作者:Sasa Wang、Xinzheng Chen、Qiaoqiao Ao、Huifei Wang、Hongbin ZhaiDOI:10.1039/c6cc03835b日期:——A new decarboxylative Csp3-Csp3 coupling approach for the benzylation of ketone enolates has been developed. A variety of raspberry ketone derivatives were conveniently synthesized in good to excellent yields under...已经开发出一种新的用于羧甲基化烯醇烯酸酯的脱羧Csp3-Csp3偶联方法。在高温条件下,可以方便地合成多种树莓酮衍生物,并具有良好的产率。
-
溴酚-吡唑啉类化合物及其合成方法和应用
表征谱图
-
氢谱1HNMR
-
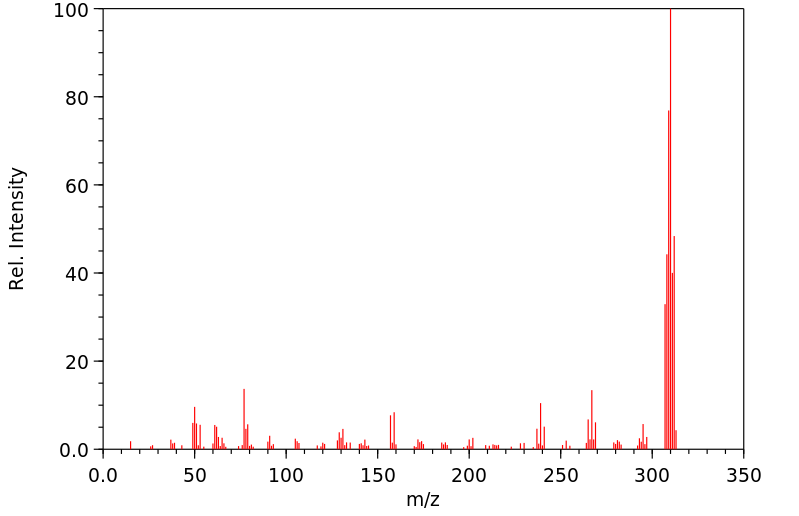
质谱MS
-
碳谱13CNMR
-
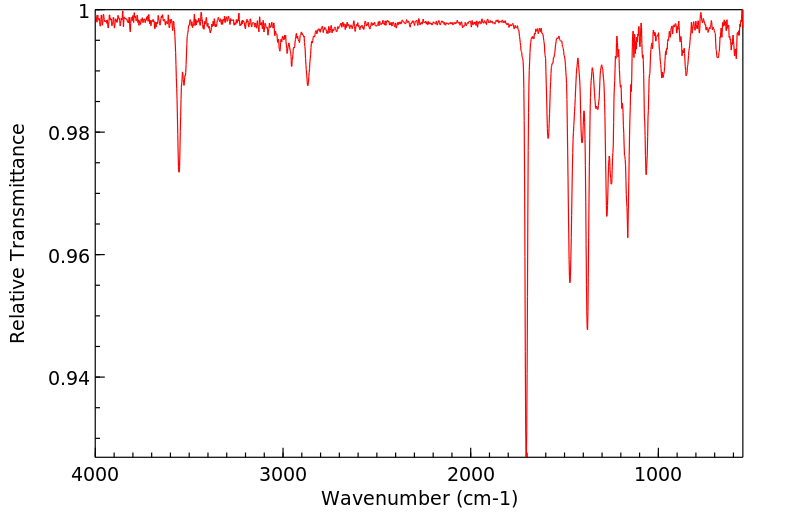
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚